Translate this page into:
Spinal Cord Ependymoma – Surgical Management and Outcome
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Ependymoma is a common primary neoplasm of the spinal cord and filum terminale. Patients with spinal ependymoma usually experience gradual symptoms due to slow progression of the tumor; thus, early diagnosis can be challenging to make.
Objective:
The objective of this study was to report 5 years’ experience in treating spinal intramedullary ependymomas and to illustrate the advantage of aggressive complete resection whenever possible.
Patients and Methods:
Retrospective medical notes of all patients with spinal ependymoma treated surgically over a 5-year period between January 2003 and January 2008 were recorded. Clinical presentation, spinal level, extent of resection, and complications were recorded. A prolonged follow-up was documented.
Results:
There were 20 patients – 11 males, and nine females –included in this study. Their median age was 48 years (range 3–75 years). In 18 patients, total gross resection was achieved. Subtotal resection was only possible in one patient due to surgical difficulty. One patient underwent biopsy and referred for further surgery and subsequently had total resection.
Conclusions:
Radical total resection is achievable in spinal ependymomas, with minimal resultant morbidity.
Keywords
Ependymoma
intramedullary
myxopapillary
spinal tumors
spine
INTRODUCTION
Ependymoma is the most common primary glial neoplasm of spinal cord representing 50%–60% of all intramedullary cord neoplasms.[12] They frequently present in middle-aged adults but can occur at any age group. Ependymomas are believed to be slow-growing tumors and exhibit benign pathology behavior. Due to the tendency to compress rather than infiltrate the adjacent parenchyma, patients with spinal or conus ependymomas mostly have insidious clinical symptomatology, and often the tumors are present long before diagnosis.[3]
Spinal cord ependymomas can be found at any spinal segment; however, they are most commonly encountered in the cervical region.[4] On the other hand, up to 90% of neoplasms that arise in the conus medullaris/cauda equina are ependymomas of myxopapillary subtype [Figure 1].[5]
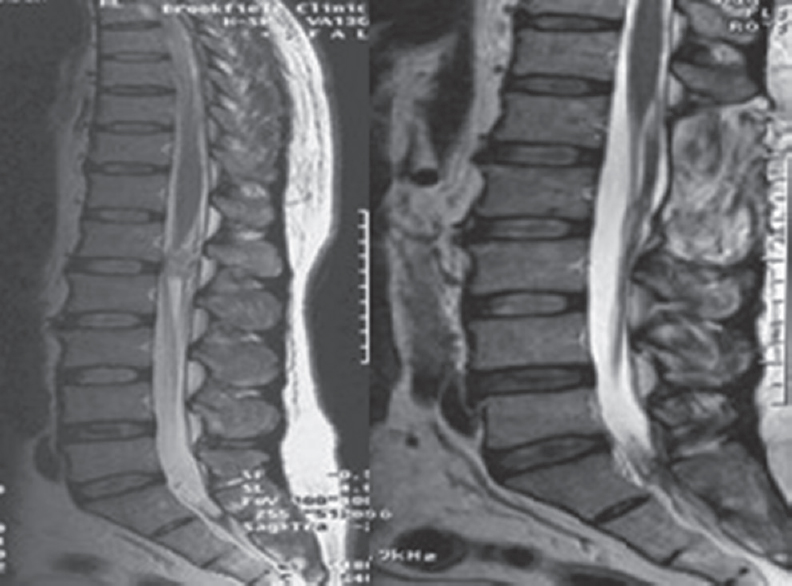
- Preoperative and postoperative T2 magnetic resonance imaging showing L1 ependymoma
Surgical resection has been advocated to these tumors given its oncological characteristics, and this has been evident in both adult and pediatric population.[67]
The surgical modality of treatment for this type of tumor has been adopted by most of the neurosurgical centers aiming for gross total resection.[89] Studies recommended that surgery should be scheduled urgently in patients with worsening neurological deficits.[9] It was also concluded that surgical resection of spinal ependymoma improves preoperative symptoms.[10]
We designed this study to analyze patients with spinal cord ependymoma in our neurosurgical unit and to report our own experience in the management of this particular surgical entity highlighting the advantage of aggressive surgical resection.
PATIENTS AND METHODS
This study is a single cohort of patients with spinal cord ependymomas operated in our hospital between January 2003 and January 2008. A retrospective evaluation of charts was conducted in this series analyzing the clinical presentation, spinal level, extent of surgery, complications, and follow-up. Data were cross-examined with the histopathological analysis results. Within the defined study period, no cases were excluded from the study.
In this study, we reported 20 patients with ependymomas of spinal cord and cauda equina. There were 11 men (55%) and 8 women (40%) and one child; the age range was 3–75 years (median of 49 years). Postoperative follow-up periods ranged from 9.8 to 15 years (median 12.4 years).
Each patient had at least one spinal magnetic resonance imaging (MRI) preoperatively and postoperatively composing of T1-weighted and T2-weighted sequences. Follow-up MRI was performed annually thereafter. All patients underwent surgery following standard informed consent under general anesthesia in the prone position. Our surgical techniques were in keeping with those described in the literature previously using posterior midline approach. Neurophysiology monitoring was only used in one patient.
For assessment of neurological function in our group, the modified McCormick classification was applied [Table 1]. We classified our patients into four pain categories ranging from absent pain to severe pain.
| I. Intact neurologically, normal ambulation, minimal dysesthesia |
| II. Mild motor or sensory deficit and functional independence |
| III. Moderate deficit and limitation of function |
| IV. Severe motor or sensory deficit, limited function, and dependent |
| V. Paraplegia or quadriplegia, even w/flickering movement |
RESULTS
Preoperative findings
On initial assessment, the most frequent presenting symptom in our series was pain (14) accounting for 70%. Three patients (15%) presented with limb paraesthesia. Bladder dysfunction was present in one case. Two patients showed objective motor weakness (10%), one patient had segmental sensory deficit, while 17 patients (85%) had normal function.
Pain was found to be axial neck (20%), mid or lower back discomfort (35%), or radicular limb pain (25%). We documented the radiological reports of initial MRI in all of our patients. Only one patient included in this series underwent previous biopsy prior referral to our institute, aiming to confirm the diagnosis.
The radiological diagnosis before surgery was reported to be ependymoma in 16 patients (80%). Astrocytoma was radiographical diagnosis in two patients; the other two were reported as meningioma.
SURGICAL RESULT
We attained gross total resection in 18 patients (90%). Subtotal removal was noted in one patient, where the surgical plane was difficult to delineate, and further resection was thought to induce neurological deficits. Subsequent postoperative radiotherapy was arranged. One patient underwent biopsy only. The latter patient was then readmitted in the same institute for complete resection after histopathology report confirmed the diagnosis of ependymoma [Figure 2]. Surgery was scheduled, and gross total resection was achieved but was not included in this series because it occurred outside our cohort.

- Smear of resected ependymoma showing moderately glial derived and focally myxoid lesion
Intraoperatively, all tumors were located in the midline except in one case where the tumor was eccentric [Figure 3].

- (a) Extensive cervical ependymoma with associated syrinx and (b) postoperative image showing total resection
This study reported no mortality related to surgery. Five patients had postoperative complications. Three patients developed respiratory tract infections. Those patients received intravenous antibiotics and encouraged to have physiotherapy. Two patients had superficial wound infections requiring antibiotic therapy and regular inspection and dressing. In these series, two patients developed postoperative deep-vein thrombosis confirmed by imaging and was commenced on anticoagulation. Median follow-up for those patients in this group was 56 months, and no recurrence was identified.
CLINICAL RESULT
Table 2 summarizes the outcome of all 20 patients included in this group, with particular attention to neurology status preoperatively and postoperatively.
| Patient | Age | Sex | Level | Surgery | Neurology Function (pre-op/post op neurology assessment) |
|---|---|---|---|---|---|
| 1 | 37 | Male | C3 | Total | I/I |
| 2 | 36 | Female | T12-L1 | Total | I/I |
| 3 | 40 | Female | T12 | Total | I/II |
| 4 | 74 | Male | T2-4 | Total | I/I |
| 5 | 26 | Male | L1-L5 | Total | I/I |
| 6 | 45 | Male | L2-S1 | Total | I/II |
| 7 | 56 | Female | T1 | Total | I/I |
| 8 | 68 | Male | C3-6 | Biopsy | I/I |
| 9 | 3 | Female | C2 | Total | I/I |
| 10 | 42 | Male | C1-T1 | Total | II/II |
| 11 | 76 | Male | C2-4 | Total | I/I |
| 12 | 38 | Female | L4 | Total | I/I |
| 13 | 53 | Male | C6-T2 | Subtotal | II/I |
| 14 | 42 | Female | C7 | Total | I/I |
| 15 | 52 | Male | L1 | Total | I/I |
| 16 | 52 | Male | L1 | Total | I/I |
| 17 | 47 | Female | L2 | Total | I/I |
| 18 | 49 | Female | T12 | Total | I/I |
| 19 | 71 | Male | T10 | Total | I/I |
| 20 | 71 | Female | T10 | Total | I/I |
Using the modified McCormack scale for assessment of neurology status of our patients preoperatively and postoperatively, conditions of 17 patients remained stable, improved in one patient, and worsened in two patients. One patient developed increasing paraesthesia postoperatively, and another patient developed marked right-sided weakness and underwent rehabilitation in our institute where she recovered partially. The latter was the only patient with eccentric tumor location, and she regained her preoperative motor function after 8 months of extensive rehabilitation. This may suggest that eccentric location of neoplasm carries poorer prognosis for resection at least in the early postoperative period.
We achieved 90% gross total resection in those cases. None of our cohort received adjuvant treatments postoperatively except for one patient in whom a subtotal resection was performed.
DISCUSSION
In our series, we report all ependymomas of spinal cord that underwent microsurgical resection in our center from January 2003 to January 2008. We documented and analyzed our patients’ operative and postoperative details. The follow-up period averaged 12.4 years.
Bagley et al. suggested that signs and symptoms usually correspond to tumor volume, invasion, and presentation site.[11] Ependymoma is known for the insidious clinical course, predominantly attributed to the tumor slow growth. Those findings are similar in our patients. Gavin Quigley et al. linked the outcome to preoperative neurological status, while the prognostic effect of symptoms, signs, and cytological features on long-term survival has been studied by other authors.[10] Analyzing the outcome regarding neurology function in our patients, we found that normal neurological preoperative status is associated with better outcome.
Published data described a surgical technique to resect intrinsic cord lesions in great details.[1112131415] In general, tumors are approached through a dorsal approach through midline myelotomy, and the strategy is to dissect in the plane between normal and neoplasm tissue. Whenever it is localized and encapsulated, the goal is usually to remove it “en bloc” in preference to piecemeal resection to prevent tumor spillage [Figure 4]. En bloc has been described to correlate strongly with less recurrence.[67]

- Picture of a large cervicodorsal ependymoma resected en bloc
This was the approach adopted in all our cases; however, due to operative difficulty and undefined surgical plane, total gross resection was not possible in one case.
Literature data suggested that gross total resection is the determining factor to achieve long-term control of the tumor.[41415] The authors believe that surgical treatment of ependymoma of spinal cord and cauda equina is safe and should always be considered before any other modality.[15] McCormick et al. published his 23 patient's study, encouraging total resection of spinal ependymomas. His 12-year-long follow-up showed low morbidity and excellent long-term control.[16] These results match our series results.
Only one case from our series received postoperative radiotherapy. Gross total resection often eliminates the need for postoperative radiotherapy. Adjuvant therapy, however, is reserved for cases of subtotal resection or in the presence of malignant ependymoma.[16171819]
Aghakhani et al., Nakamura et al., Raco et al., and Lin et al. reported their spinal ependymoma series. They achieved a total gross resection of 91.5%, 91%, 81%, and 65%, respectively.[20212223] Further results from Kaner et al. published his figures of 100% total resection over 14 years.[13]
Perioperatively, neurophysiologic monitoring with somatosensory-evoked potentials was not utilized frequently in our spinal ependymoma series.
Ependymoma mostly exhibits benign slow-growing course; nevertheless, they carry a risk of recurrence despite maximum gross resection.[4] Continuous clinical and radiological surveillance is recommended. Annual gadolinium-enhanced MRI study is the investigation of choice to screen and rule out recurrence.[1324] Recurrence was found to correlate strongly with subtotal and piecemeal resections.[25]
We encountered no tumor recurrence during radiological and clinical follow-up that averaged 12.4 years, documented with every clinic visit and whenever follow-up radiological studies have been requested and performed.
CONCLUSIONS
We studied a large series of spinal cord ependymoma with an extensive follow-up period.
To attain an excellent outcome, early diagnosis and treatment are crucial. Radical gross total resection is the aim in most of the spinal ependymomas.
As we have described, good surgical results are achievable through radical resection with minimal resultant morbidity.
Thorough clinical along with regular radiological follow-up is essential in ependymoma patients. Radiation therapy is recommended as surgical adjunct and reserved to subtotal resection cases, disseminated, or malignant disease.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Outcome of surgery for intramedullary spinal ependymoma. Ann Saudi Med. 2008;28:109-13.
- [Google Scholar]
- Primary intraspinal neoplasms in Norway, 1955 to 1986. A population-based survey of 467 patients. J Neurosurg. 1989;71:842-5.
- [Google Scholar]
- Giant cell ependymoma of the spinal cord. Case report and review of the literature. J Neurosurg. 2004;100:75-9.
- [Google Scholar]
- Surgical outcome and prognostic factors of spinal intramedullary ependymomas in adults. J Neurooncol. 2002;57:133-9.
- [Google Scholar]
- Spinal myxopapillary ependymoma: Neurological deterioration in patients treated with surgery. Spine (Phila Pa 1976). 2009;34:1619-24.
- [Google Scholar]
- Gross total resection rates of grade II/III intramedullary ependymomas using the surgical strategy of en-bloc resection without intra-operative neurophysiological monitoring. Br J Neurosurg. 2017;31:364-8.
- [Google Scholar]
- Predictors of functional outcome after spinal ependymoma resection. J Neurosci Rural Pract. 2018;9:354-8.
- [Google Scholar]
- Results of microsurgical treatment for intramedullary spinal cord ependymomas: Analysis of 36 cases. Neurosurgery. 1999;44:264-9.
- [Google Scholar]
- Spinal cord ependymoma: Radical surgical resection and outcome. Neurosurgery. 2002;51:1162-72.
- [Google Scholar]
- Outcome predictors in the management of spinal cord ependymoma. Eur Spine J. 2007;16:399-404.
- [Google Scholar]
- Myxopapillary ependymoma. A clinicopathologic and immunocytochemical study of 77 cases. Cancer. 1985;56:883-93.
- [Google Scholar]
- Clinical analysis of 21 cases of spinal cord ependymoma: Positive clinical results of gross total resection. J Korean Neurosurg Soc. 2010;47:102-6.
- [Google Scholar]
- Prevention of postoperative posterior tethering of spinal cord after resection of ependymoma. J Neurosurg. 2003;99:181-7.
- [Google Scholar]
- Spinal cord ependymoma: Radical surgical resection and outcome. Neurosurgery. 2003;53:246.
- [Google Scholar]
- Adult intramedullary spinal cord ependymomas: The result of surgery in 38 patients. J Neurosurg. 1993;79:204-9.
- [Google Scholar]
- Postoperative radiotherapy in the management of spinal cord ependymoma. J Neurosurg. 1991;74:720-8.
- [Google Scholar]
- Spinal ependymomas – The value of postoperative radiotherapy for residual disease control. Br J Neurosurg. 1996;10:559-66.
- [Google Scholar]
- Intramedullary spinal ependymomas: Analysis of a consecutive series of 82 adult cases with particular attention to patients with no preoperative neurological deficit. Neurosurgery. 2008;62:1279-85.
- [Google Scholar]
- Surgical treatment of intramedullary spinal cord tumors: Prognosis and complications. Spinal Cord. 2008;46:282-6.
- [Google Scholar]
- Long-term follow-up of intramedullary spinal cord tumors: A series of 202 cases. Neurosurgery. 2005;56:972-81.
- [Google Scholar]
- Treatment of spinal cord ependymomas by surgery with or without postoperative radiotherapy. J Neurooncol. 2005;71:205-10.
- [Google Scholar]
- Spinal cord ependymoma: Contribution of MRI using gadolinium. Rev Neurol (Paris). 1990;146:228-30.
- [Google Scholar]
- Outcomes following myxopapillary ependymoma resection: The importance of capsule integrity. Neurosurg Focus. 2015;39:E8.
- [Google Scholar]






