Translate this page into:
Extended Window for Stroke Thrombectomy
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
Mechanical thrombectomy is the standard treatment for large vessel occlusion (LVO) in acute ischemic stroke (AIS) up to 6 h after onset. Recent trials have demonstrated a benefit for wake-up strokes and patients beyond 6 h.
Methods:
A systematic literature review was conducted for multicenter randomized clinical trials (RCTs) investigating endovascular stroke treatment using perfusion imaging to identify patients that may benefit from mechanical thrombectomy for AIS beyond 6 h of onset. Random effects meta-analysis was used to analyze the following outcomes: 90-day functional independence rates with modified Rankin Scale (mRS ≤2), 90-day mortality, and symptomatic intracranial hemorrhage (sICH) rates. Further stratification was carried out by age and presentation.
Results:
Two multicenter RCT's were included as follows: DAWN and DEFUSE-3. Pooled 90-day functional independence rates favored endovascular management (odds ratio [OR] 5.01; P < 0.00001). Subgroup analysis demonstrated continued 90-day functional independence benefit for endovascular management regardless of age (≥80 years, OR 5.65, P = 0.01; ≤80 years, OR 4.92, P < 0.00001). When stratified for the manner of stroke discovery, 90-day functional independence rates favored endovascular management for wake-up strokes (OR 8.74, P < 0.00001) and known-time onset strokes (OR 5.08, 95% confidence interval [CI] 2.04–12.65, P = 0.0005), although no benefit was observed for unwitnessed strokes (OR 1.64, 95% CI 0.17–16.04, P = 0.67). No difference observed in 90-day mortality rates (OR 0.71; P = 0.14) or in SICH rates (OR 1.67; P = 0.29).
Conclusions:
This meta-analysis reinforces that endovascular management is superior to standard medical management alone for the treatment of AIS due to LVO beyond 6 h of onset in patients with perfusion-imaging selection.
Keywords
DAWN
DEFUSE
ischemic stroke
perfusion
thrombectomy
INTRODUCTION
Stroke is the fourth leading cause of mortality and the leading cause of long-term disability in the United States, affecting approximately 795,000 people each year.[1] The majority of these strokes are ischemic (87%). In 1995, the use of intravenous tissue plasminogen activator (IV-tPA) to treat acute ischemic stroke (AIS) within 4.5 h from symptom onset became the standard of care.[2] Subsequently, in 2015, five randomized clinical trials (RCTs) demonstrated the significant clinical benefit of endovascular mechanical thrombectomy for AIS due to large vessel occlusion (LVO) over standard medical therapy, when performed within 6 h of symptom onset.[3456789]
The national guidelines and consensus statements in the United States, Europe, and Canada have recommended endovascular recanalization up to 6 h of stroke onset.[10] However, the time of stroke onset is typically defined as the time the patient was last known to be well, which is often uncertain. The results of the recently reported DAWN (DWI or CTP Assessment with Clinical Mismatch in the Triage of Wake-Up and Late Presenting Strokes Undergoing Neurointervention with Trevo) as well as DEFUSE-3 (Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke 3) trials suggest that the window for endovascular treatment may be extended up to 24 h for selected patients based on imaging and clinical examination.[1112] Results of these studies indicate that patients with ischemic brain tissue that has not yet undergone infarction, or with penumbra, have improved functional outcomes with endovascular intervention.
This meta-analysis aims to compare the rates of functional independence, mortality, and symptomatic intracranial hemorrhage (sICH) between endovascular and medical management for AIS due to LVO beyond 6 h of onset of symptoms. In addition, we will investigate if these results are contiguous among specified subgroups. We hypothesize that the combined data will show consistent safety and efficacy in those receiving endovascular therapies with standard medical therapy.
METHODS
This study follows the guidelines set forth by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. No registered review protocol was utilized for this study.
Study design and inclusion criteria
We performed a systematic literature search of PubMed for RCT's investigating endovascular stroke treatment published between 2017 and 2018. Studies using perfusion imaging to identify patients that may benefit from mechanical thrombectomy for AIS beyond 6 h of onset of symptoms were included in the study [Figure 1]. Two trials fit these criteria: DAWN and DEFUSE-3 trials. Available study demographic, baseline clinical, and radiographic variables were extracted. This included study trial period, inclusion/exclusion criteria, and number and location of centers that contributed. Additional information extracted included patient mean or median age, mean or median presenting National Institute of Health Stroke Scale (NIHSS), average volume of ischemic core, number of AIS treated with unknown onset, or awaking strokes. Procedural details extracted included general anesthesia (GA) usage, thrombectomy device details, and IV-tPA administration. Outcome data included successful revascularization rates, defined as modified Thrombolysis in Cerebral Ischemia Grade 2b or 3, modified Rankin Score (mRS) at 90-days poststroke, mortality rates, and symptomatic intracranial hemorrhage (sICH) rates.
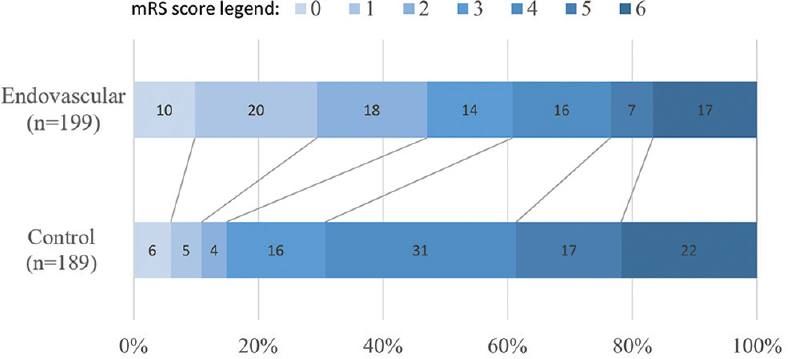
- Pooled DAWN and DEFUSE-3 modified Rankin Score (mRS) scores. Numbers represent percentages of patients in each outcome group. mRS range is 0–6: 0 indicating no symptoms, 1 no clinical disability, 2 slight disability, 3 moderate disability, 4 moderately severe disability, 5 severe disability, and 6 death. Percentages are rounded to the nearest whole number
Statistical analysis
Descriptive statistics were determined using SAS version 9.4 (Cary, NC). The pooled data analysis was done with Review Manager Version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration). Odds ratio (OR) for the studies were calculated using the Mantel–Haenszel test. A random effects model was utilized versus fixed-effects model, to account for sampling and inclusion variation between the studies. Using the Chi-square and I2 test statistics, we checked for study homogeneity, with significant heterogeneity determined when both χ2 was 10% significant and I2 was larger than 50%. Statistical tests were two-sided and P < 0.05 was considered statistically significant. Assessment of study bias was performed using the Grading of Recommendations, Assessment, Development, and Evaluation guidelines.[13]
RESULTS
Study selection
The literature search produced two multicenter prospective RCT investigating endovascular treatments versus standard medical management for AIS due to LVO beyond 6 h: DAWN and DEFUSE-3. Together, these two investigations contributed 388 patients to this meta-analysis [Table 1].[1112] The two trials demonstrated low risks for selection, detection, and reporting bias [Table 2]. Both trials had a considerable risk of performance bias, as participants and practitioners were not blinded to treatment. The DAWN trial was halted following and interim analysis reaching a prespecified criterion for superiority. The DEFUSE-3 trial was halted at prematurely at 182 patients before the first prespecified interim analysis because of the publication of DAWN results, leaving it susceptible to attrition bias. In addition, both trials contained intervention protocol violations in a few subjects. Specifically, in DAWN, three patients underwent treatment with unauthorized devices; in DEFUSE-3, two patients were treated with intracranial stent placement, two patients with intra-arterial tissue plasminogen activator, and one patient with angioplasty.
| Trial | DAWN | DEFUSE-3 | |
|---|---|---|---|
| Publication year | 2017 | 2018 | |
| Enrollment Criteria | Trial period | September 2014- February 2017 | May 2016- May 2017 |
| Location | United States, Canada, Australia | United States | |
| No. of Centers | 26 | 38 | |
| No. of Patients | 206 | 182 | |
| Last known well to randomization time, h | ≥ 6 and≤24 | ≥ 6 and ≤16 | |
| Age, years | ≥18 | 18-90 | |
| NIHSS score | ≥ 10 | ≥ 6 | |
| Perfusion inclusion | (1) < 1/3 MCA territory, | (1) Ischemic core ≤70 ml | |
| (2) < 21 ml core infarct, when NIHSS≥10 and age ≥80 y | (2) Ratio of volume of Ischemic tissue to infarct volume ≥1.8 | ||
| (3) < 31 ml core infarct when NIHSS ≥10 and age <80 y | (3) Penumbral tissue ≥15 ml | ||
| (4) 31 ml to <51 ml core infarct when NIHSS ≥20 and age <80 y | |||
| Endovascular Intervention | Stent Retrieval with Trevo device (Stryker Neurovascular) | Any FDA approved device | |
| Primary endpoint | Mean utility-weighted mRS at 90 d* | mRS at 90 d |
| Trial | DEFUSE-3 | DAWN | Total | |
|---|---|---|---|---|
| Intervention Arm | ITT patients, n | 92 | 107 | 199 |
| Stent Retriever, n (%) | 74 (80) | 102 (95) | 176 (88) | |
| Aspiration Alone, n (%) | 25 (27) | 0 (0) | 25 (13) | |
| IV-tPA, n (%) | 10 (11) | 5 (5) | 15 (8) | |
| Median NIHSS | 16 | 17 | ||
| Median/mean age, y | 70 | 69.4 | ||
| Wake-up strokes, n (%) | 49 (53) | 67 (63) | 116 (58) | |
| Median Infarct/Ischemic core, ml | 9.4 | 7.6 | ||
| GA, n (%) | 26 (28) | 11 (10) | 37 (19) | |
| Median time, last well to randomization, hr | 10.88 | 12.2 | ||
| Control Arm | ITT patients, n | 90 | 99 | 189 |
| IV-tPA, n (%) | 8 (9) | 13 (13) | 21 (11) | |
| Median NIHSS | 16 | 17 | ||
| Median/mean age, y | 71 | 70.7 | ||
| Wake-up strokes, n (%) | 42 (47) | 47 (47) | 89 (47) | |
| Median Infarct/Ischemic core, ml | 10.1 | 8.9 | ||
| Median time, symptom to randomization, hr | 10.73 | 13.3 |
Demographics and study characteristics
Table 1 summarizes the designs and relevant inclusion criteria of included studies. The DAWN trial was published in November 2017 and DEFUSE-3 in January 2018. The aggregate participating centers between the two studies were 64. Key differences between the studies inclusion criteria are summarized in Table 1. Table 3 outlines the characteristics of both study's control and intervention arms. When combined, intention-to-treat patients in the intervention and control arms included 199 and 189 patients, respectively. IV-tPA was administered in 15 patients (8%) in the intervention arm and 21 patients (11%) in the control arm. DAWN investigators were limited to the Trevo stent retriever (Stryker Neurovascular) while DEFUSE-3 investigators could utilize any number of Food and Drug Administration approved stroke thrombectomy devices. Overall, a stent retriever was utilized in 176 patients (88%), and 25 patients (13%) had aspiration thrombectomy alone. Median infarct/ischemic core in the intervention arms was 9.4 ml (interquartile range [IQR] 2.3–25 ml) and 7.6 (IQR 2–18 ml) for DEFUSE-3 and DAWN, respectively; control arm infarct core size was similar. The median NIHSS in intervention arms was 16 (IQR 10–20) and 17 (IQR 13–21) for DEFUSE-3 and DAWN, respectively. Aggregate number of wake-up strokes in the intervention and control groups was 116 (58%) and 89 (47%), respectively. In the intervention arm, GA was administered in 37 patients (19%).
| mRS | Trial | Total, n (%) | ||
|---|---|---|---|---|
| DEFUSE-3 | DAWN | |||
| Control arm: mRS scores last F/u | 0 | 9 | 10 | 19 (10) |
| 1 | 15 | 24 | 39 (20) | |
| 2 | 17 | 18 | 35 (18) | |
| 3 | 14 | 14 | 28 (14) | |
| 4 | 17 | 14 | 31 (16) | |
| 5 | 7 | 7 | 14 (7) | |
| 6 | 13 | 20 | 33 (17) | |
| Total, n | 92 | 107 | 199 | |
| 0 | 7 | 4 | 11 (6) | |
| Intervention arm: mRS scores last F/u | 1 | 4 | 5 | 9 (5) |
| 2 | 4 | 4 | 8 (4) | |
| 3 | 14 | 16 | 30 (16) | |
| 4 | 24 | 34 | 58 (31) | |
| 5 | 14 | 18 | 32 (17) | |
| 6 | 23 | 18 | 41 (22) | |
| Total, n | 90 | 99 | 189 | |
| Primary endpoint result | Endovascular superior, OR 2.77 (CI 1.6 to 4.70) | Endovascular superior, 5.5 vs 3.4 (thrombectomy vs medical)* | ||
| mTICI of 2b or 3 in intervention | 74 of 92 | 90 of 107 | 164 of 199 (82%) | |
Outcomes and complications of endovascular versus medical management
In the endovascularly managed group, successful revascularization, defined as modified thrombolysis in cerebral infarction of 2b or 3, was achieved in 164 patients (82%) between both studies. Figure 1 summarizes the 90-day poststroke mRS scores. A 90-day mRS ≤3 was observed in 60.8% of patients (121 of 199) in the endovascular group, compared with 30.7% (58 of 189) in the control group. Conversely, a 90-day mRS ≥4 was observed in 39.2% of patients (78 of 199) in the endovascular group, compared with 69.3% (131 of 189) in the control group.
Meta-analysis of pooled data from the DAWN and DEFUSE-3 trials can be seen in Figure 2, showing favorable 90-day functional independence (mRS 0–2) in the endovascular-treated patients versus the control group (46.7% vs. 15%; OR 5.01; 95% confidence interval [CI] 3.07–8.17; P < 0.00001). No significant heterogeneity between the studies, for 90-day functional independence, was observed (χ2 = 0.78; P = 0.38; I2 = 0%).

- Functional independence (mRS 0–2), mortality (mRS 6) at 90 days, and sICH following endovascular or medical management of acute ischemic stroke due to large vessel occlusion. Forest plot of odds ratios for (a) functional independence (modified Rankin score or mRS 0–2) at 90 days, (b) all-cause mortality at 90-day, (c) and symptomatic intracerebral hemorrhage (sICH). Estimated odds ratios and confidence intervals are shown, respectively, by the square box and horizontal line. The combined studies odds ratio and confidence interval are in bold and shown by the horizontal diamond. Heterogeneity tests and effect size are shown
No significant difference of 90-day mortality between intervention and control was revealed with the pooled data (OR 0.71; 95% CI 0.34–1.02; P = 0.14). No significant heterogeneity between the studies, for 90-day mortality, was observed (χ2 = 2.13; P = 0.38; I2 = 53%). Similarly, no significant difference was observed in sICH rates (OR 1.67; 95% CI 0.64–4.35; P = 0.29). No significant heterogeneity between the studies, for sICH rates, was observed (χ2 = 0.06; P = 0.81; I2 = 0%).
Pooled subgroup analysis for 90-day functional independence after intervention versus control
Figure 3 shows the pooled subgroup analyses performed. When stratified for age 80 years or older, there was still a significant overall benefit in 90-day functional independence (mRS 0–2) for endovascular management (≥80 years, OR 5.65, 95% CI 1.44–22.12, P = 0.01; ≤80 years, OR 4.92, 95% CI 2.87–8.44, P < 0.00001). This benefit was not significantly evident in patients older than 80 years in the DEFUSE-3 trial alone. There was no significant heterogeneity between the studies respective age subgroups.

- Subgroup analysis of functional independence (mRS 0–2) following endovascular or medical management of acute ischemic stroke due to large vessel occlusion. Forest plot of odds ratios for functional independence (modified Rankin score or mRS 0–2) at 90 days stratified into subgroups: (a) Age, 80 or older and (b) Manner of stroke discovery: Wake-up stroke, unwitnessed, or time-known. Estimated odds ratios and confidence intervals are shown, respectively, by the square box and horizontal line. The combined studies odds ratios and confidence interval are in bold and shown by the horizontal diamond. Heterogeneity tests and effect size are shown
When stratified into stroke onset type (i.e., wake-up, unwitnessed, and time-known strokes), a 90-day functional independence (mRS 0–2) benefit was observed in endovascular-treated patients for wake-up strokes (OR 8.74, 95% CI 3.87–19.75, P < 0.00001) and time-known strokes (OR 5.08, 95% CI 2.04–12.65, P = 0.0005); however, there was no significant pooled benefit seen for unwitnessed strokes (OR 1.64, 95% CI 0.17–16.04, P = 0.67). For both wake-up stroke and time-known stroke subgroups, there was no significant study heterogeneity observed, although there was significant heterogeneity seen for the unwitnessed stroke subgroup (χ2 = 4.11; P = 0.04; I2 = 76%).
DISCUSSION
In this meta-analysis of pooled study data from both the DAWN and DEFUSE-3 trial, we show that endovascular therapy with medical therapy is five times more likely to result in 90-day functional independence when compared with medical treatment alone, in select patients suffering AIS due to LVO beyond 6 h of onset. In addition, we confirm that there is no significant difference in 90-day mortality and sICH rates between intervention and medical management alone.
Both included studies were assessed for heterogeneity, qualitatively and quantitatively. The unwitnessed stroke type was the only subgroup that demonstrated significant study heterogeneity. Due to both its lower NIHSS and larger ischemic core inclusion criteria, the DEFUSE-3 study covered a broader patient population, possibly accounting for the slight decrease in 90-day functional independence benefit seen when compared to the DAWN trial.
DEFUSE-3 had a higher GA administration than DAWN (28% vs. 10%). In stroke thrombectomy literature, it is uncertain whether GA or conscious sedation (CS) is superior. Pooled data from the HERMES collaborators and the 2015 trials found that GA for mechanical thrombectomy resulted in lower rates of 90-day functional independence when compared with patients who received CS.[414] However, the HERMES data were extracted from studies that were not designed or randomized to test the superiority of GA versus CS. Schönenberger et al. performed a dedicated RCT to determine which is favorable, finding that patients under GA, during thrombectomy, had higher 90-day functional independence rates compared with patients under CS (37.0% in GA vs. 18.2% in CS; P = 0.01).[15] Therefore, different rates of GA administration may confound the results drawn by the DAWN and DEFUSE-3 studies.
Saver et al. found that increased stroke onset to endovascular treatment time is associated with worse outcomes.[16] When compared with the pooled data taken from the five major 2015 thrombectomy trials, the combined 90-day functional independence rates for the intervention arms were higher in the late symptom onset to treatment trials, i.e., DAWN and DEFUSE-3 (57% vs. 46%). From the 2015 studies, two had perfusion-imaging inclusion criteria (SWIFT-PRIME and EXTEND-IA).[69] When combined, the intervention groups from SWIFT-PRIME and EXTEND-IA studies had a 65% (87 of 133 patients) 90-day functional independence rate. These superior results observed with perfusion imaging suggest that it may have a significant role in future thrombectomy patient selection regardless of time of stroke onset. When comparing the control arms of the 2015 trials and the DAWN and DEFUSE-3 trials, the opposite relationship is observed, with worse functional independence in the DEFUSE-3 and DAWN trials (15% vs. 26.5%).[4] This is likely attributable to the latent temporality differences between the inclusion criteria of the two sets of studies leading to a decreased administration of IV-tPA in patients in the DAWN and DEFUSE-3 studies compared with the 2015 trials (11% vs. 87%).
The subgroup analysis of the pooled data from the 2015 trials demonstrated that older age was correlated with a higher 90-day mRS score following endovascular intervention; however, treatment effect remained constant regardless of age. Furthermore, the elderly thrombectomy group (≥80 years) was the only age group to show a slightly decreased risk of death.[4] Real-world studies have found different results. STRATIS investigators found that, in the real-world setting, functional independence rates decreased significantly for each 5-year increment of age (<65–>90 years; 64.3%–26.5%), while mortality increased (7.9%–35.1%); additional real-world retrospective studies produced similar results.[171819] In solidarity, the DEFUSE-3 trial was unable to establish a significant benefit for patients older than 80 years; however, aggregate DAWN and DEFUSE-3 data showed overall significant benefit for this patient population. Considering the similar enrollment of elderly patients (≥80 years), the lack of significant benefit in the DEFUSE-3 trial may suggest that older patients with large infarct volumes may fair worse following thrombectomy when compared to younger patients with similar imaging, due to DEFUSE-3's broader inclusion criteria. Further investigation is needed to stratify the benefits of endovascular intervention in the elderly population with regard to perfusion measured infarct size; an aim that DAWN was initially designed to achieve, though failed to reach adequate power to perform analysis due to early termination.
When examining pooled 90-day functional independence rates stratified by the manner of stroke discovery, we found the continued benefit of endovascular therapy in wake-up and time-known strokes; however, we failed to observe a significant benefit for endovascular therapy in the unwitnessed strokes. When the trials are assessed separately for unwitnessed stroke benefit, the DAWN trial found endovascular superiority while DEFUSE-3 did not. This was likely due to the modest number of patients, in that category, enrolled in the DEFUSE-3 trial. Moreover, significant study heterogeneity in the unwitnessed stroke subgroup leaves the pooled analysis flawed.
Limitations
The DAWN and DEFUSE-3 trials are not without limitations. Both trials were terminated early, DAWN after reaching a prespecified definition of superiority at an interim analysis and DEFUSE-3 after NIH requested trial termination following DAWN trial publication. In both studies, the practitioners and patients were unblinded to treatment modality, though there was a blind assessment of outcome measures. In addition, both trials had device protocol violations; DAWN had three and DEFUSE-3 had five violations. All the centers included in the studies were experienced stroke centers, thus results still need to be tested for generalizability in less experienced centers.
Our meta-analysis is limited by the pooled data available from the included studies, a common limitation among meta-analyses. In addition, we are unable to account for the differences in study inclusion/exclusion criteria and devices permitted for endovascular intervention. Furthermore, both studies required LVO located in either internal carotid artery or proximal (M1) division of the middle cerebral artery, thus the findings are not necessarily generalizable to the distal anterior circulation or the posterior circulation.
CONCLUSIONS
This meta-analysis from multicenter RCTs comparing endovascular management with medical management alone for AIS due to LVO beyond 6 h of onset demonstrated significantly higher 90-day functionality rates in the endovascularly managed patients. Patients older or younger than 80 years and wake-up or time-known strokes continued to demonstrate a higher rate of functional independence in the endovascular group. This meta-analysis reinforces that endovascular management paired with standard medical management is superior to standard medical management alone for the treatment of AIS due to LVO beyond 6 h of onset in patients with perfusion-imaging selection.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Heart disease and stroke statistics-2016 update: A report from the American Heart Association. Circulation. 2016;133:e38-360.
- [Google Scholar]
- Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581-7.
- [Google Scholar]
- Endovascular vs medical management of acute ischemic stroke. Neurology. 2015;85:1980-90.
- [Google Scholar]
- Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723-31.
- [Google Scholar]
- A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11-20.
- [Google Scholar]
- Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009-18.
- [Google Scholar]
- Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019-30.
- [Google Scholar]
- Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296-306.
- [Google Scholar]
- Stent-retriever thrombectomy after intravenous t-PA vs.t-PA alone in stroke. N Engl J Med. 2015;372:2285-95.
- [Google Scholar]
- Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870-947.
- [Google Scholar]
- Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11-21.
- [Google Scholar]
- Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708-18.
- [Google Scholar]
- GRADE guidelines: 4. Rating the quality of evidence – Study limitations (risk of bias) J Clin Epidemiol. 2011;64:407-15.
- [Google Scholar]
- Effect of general anaesthesia on functional outcome in patients with anterior circulation ischaemic stroke having endovascular thrombectomy versus standard care: A meta-analysis of individual patient data. Lancet Neurol. 2018;17:47-53.
- [Google Scholar]
- Effect of conscious sedation vs. general anesthesia on early neurological improvement among patients with ischemic stroke undergoing endovascular thrombectomy: A randomized clinical trial. JAMA. 2016;316:1986-96.
- [Google Scholar]
- Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: A Meta-analysis. JAMA. 2016;316:1279-88.
- [Google Scholar]
- Efficacy and safety of mechanical thrombectomy in older adults with acute ischemic stoke. J Am Geriatr Soc. 2017;65:1816-20.
- [Google Scholar]
- Outcome of mechanical thrombectomy in the very elderly for the treatment of acute ischemic stroke: The real world experience. Acta Radiol Open. 2015;4:2058460115599423.
- [Google Scholar]
- Systematic evaluation of patients treated with neurothrombectomy devices for acute ischemic stroke: Primary results of the STRATIS registry. Stroke. 2017;48:2760-8.
- [Google Scholar]






