Translate this page into:
Timed Vibration Sense and Joint Position Sense Testing in the Diagnosis of Distal Sensory Polyneuropathy
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Distal sensory polyneuropathy (DSP) is one of the most common neurological disorders. Although several studies have studied the role of the neurological examination in DSP, there are only limited studies on the utility of timed vibration sense (VBS) and joint position sense (JPS) testing in the diagnosis of DSP.
Objectives:
The objective is to study the utility of timed VBS testing and JPS testing at the great toe in clinical detection of DSP.
Methods:
This study was prospectively conducted in the neurology department of a tertiary care hospital in India. Patients with DSP referred to the electrophysiology laboratory from August 2017 to December 2017 were screened. Patients with symptomatic DSP which was confirmed by electrophysiological studies were taken as cases and normal participants with no symptoms or electrophysiological findings suggestive of DSP served as controls.
Results:
We studied 127 patients and 194 controls. The mean age of the patients was 48.7 (14.5) years in the patient group and 39.7 (14.5) years in the control group. The male: female ratio was 77/50 in the patient group and 112/82 in the control group. Abnormal clinical examination was found in 95% of the patients with DSP. The most common abnormal examination components were impaired ankle reflexes (70%), vibration (85%), and JPS (39.6%) sensation. Using the receiver operating characteristic curve for the diagnosis of DSP, a vibratory response lasting <8 s at the great toe had a sensitivity of 85% and specificity of 42.8%. For JPS testing at the great toe, obtaining two or more incorrect responses had a sensitivity of 33% and specificity of 87.6%.
Conclusion:
VBS testing was more sensitive and JPS testing was more specific in making a clinical diagnosis of DSP. For timed VBS, duration of >8 s at the great toe was a useful test to rule out DSP, and for JPS testing at the great toe, obtaining two or more incorrect responses was a useful test in ruling in the diagnosis of DSP.
Keywords
Clinical testing
joint position sense
polyneuropathy
vibration sense
INTRODUCTION
Distal sensory polyneuropathy (DSP) is a disease of the peripheral nerves and has a prevalence of 2.1%–8% in the general population.[1] Diabetes mellitus, impaired glucose tolerance, alcohol use, B12 deficiency, paraproteinemia, inherited conditions, and drugs contribute to form the more common causes of DSP.[2] DSP commonly presents with distal symmetric sensory symptoms and rarely with imbalance while walking.[3] Patients with DSP that predominantly affects the large sensory fibers (A-beta somatic fibers), present with tingling paresthesias and have impairment of joint position sense (JPS) and vibration sense (VBS) with absent ankle jerks.[4] On electrophysiological testing, the sensory nerve action potentials (SNAPs) are reduced or absent. Patients with DSP restricted to small nerve fibers (A-delta fibers and unmyelinated C fibers), have minimal findings on the neurological examination, such as reduced pinprick or temperature sensation, preserved ankle jerks, and normal nerve conduction studies.[5] Despite advances in the available technology, neurological examination continues to play an important and pertinent role in the evaluation of patients with neurological illness. However, there are limited data on its utility, and hence, there is a need for more research in this field.[6] Several studies have explored the role of the neurological examination in DSP, but these were mostly restricted to specific subgroups of patients such as diabetic polyneuropathy.[78] Very few studies have explored the correlation between symptoms, examination findings, and electrophysiological results.[7910] Timed VBS and JPS are integral parts of the sensory clinical examination, but there are limited data on its diagnostic utility.[101112] Hence, the objective in this study was to study the utility of joint position and timed VBS testing in the diagnosis of DSP.
METHODS
This was a prospective study conducted in the neuroscience department of a tertiary care hospital in India. The study was approved by the Institutional Review Board and Ethics Committee. Between the periods of August 2017 and December 2017, consecutive patients who presented to the electrophysiology laboratory of the neuroscience department with a clinical diagnosis of a DSP were screened, and those patients who were between 18 and 80 years of age and electrophysiological studies confirmed DSP were included in the patient group. Patients with acute neurological disorders, joint pathologies, disorders affecting consciousness, or awareness making them unable to cooperate for the examination and patients with skin lesions were excluded from the study. Normal participants who were willing to undergo nerve conduction studies and patients with nonneurological conditions and a normal nerve conduction study were included as the control group. The principal investigators who performed the tests for the study were blinded to the allotment of the groups. Participants of both the groups underwent timed VBS and JPS testing.
Timed vibration sense testing
A standardized tuning fork (128 Hz) was used to produce the vibration stimulus at the distal interphalangeal joint of the thumb and great toe, medial malleolus, and tibial tuberosity bilaterally.[1013] The examiner struck the tuning fork against the palm of the free hand just hard enough so that the metal ends meet, producing a metallic clanging sound. The examiner then held the stem of the tuning fork with two fingers, similar to gripping a dart so as not to touch the vibrating tines.[11] The time of starting of the VBS and time of cessation were noted using a stopwatch.
Joint position sense testing
The JPS was assessed by flexion and extension of the great toe. The great toe was lightly grasped at the sides using the thumb and index finger and moved up or down randomly by at least 20°, and the participant was asked to detect and report the direction of movement.[14] A total of 10 trials were done, and the number of correct trials was recorded.
Electrophysiological testing
The SNAPs of the median, sural, and superficial peroneal nerves were recorded using the Nicolet EDX EMG system (Natus Medical Incorporated, San Carlos, CA, USA). For testing, the participant was seated comfortably in a chair with the hand and forearm resting on a pillow. Before the placement of electrodes, the application sites were thoroughly cleaned with alcohol. The laboratory temperature was maintained at 22°C ± 2°C. SNAPs were recorded orthodromically in the median nerve and antidromically in the sural and superficial peroneal nerves. The filter settings for the sensory nerve conduction study were 10–2 kHz, sweep speed of 1–2 ms/division, and gain 1–5 mV/division. Supramaximal stimulation was set to 120%, and five readings of each nerve were obtained and averaged.[15] The amplitude of the SNAP was the vertical distance from baseline to the negative peak; latency was the time from peak of stimulus artifact to the negative peak of the waveform; and conduction velocity was the distance between stimulus and recording electrodes divided by latency.[16] Median nerve SNAPs were recorded orthodromically by placing the recording, surface electrodes 3 cm proximal to the distal crease, and reference electrode at a distance of 3 cm proximal to the recording electrode. For stimulation, a standardized stimulator was used to stimulate the second or third digits.[1617] Sural nerve SNAPs were recorded by placing surface electrodes between the lateral malleolus and Achilles tendon. The nerve was stimulated antidromically 10–13 cm proximal to the recording electrode, distal to the lower border of the gastrocnemius at the junction of middle and lower third of the leg.[171819] The SNAPs in superficial peroneal nerve were recorded by placing the active electrode just above the junction of the lateral third of a line connecting the malleoli and reference electrode 3 cm distal to it. Antidromic surface stimulation was carried out 10–13 cm proximal to the upper edge of the lateral malleolus anterior to the peroneus longus.[1920]
RESULTS
A total number of 321 participants (189: male, 132: female) were recruited to the study. A total of 127 participants had DSP as evidenced by symptoms, neurological examination, and electrophysiological evaluation (abnormal lower limb SNAPs). One hundred and ninety-four participants without any neurological deficits and normal SNAPs were taken as controls. Among patients with DSP, abnormal clinical examination was found in 95%. The abnormal findings on the baseline neurological examination included impaired sensation over the feet 66%, impaired ankle jerk (70%), impaired VBS (85%), and impaired JPS (39.6%).
The normative data of the VBS testing from the controls are given in Table 1. Joint position testing done in the controls had the number of mean correct values as 9.5 out of 10 trials with a standard deviation of 1.2. One of the normal participants aged 65 years had absent JPS at the great toe. None of the normal participants had absence of the VBS at the great toe. The clinical and electrophysiological characteristics of the patients with DSP and the normal controls are given in Table 2. Diabetes mellitus was the most common (38.6%) etiology for DSP. The diagnostic accuracy of timed VBS and JPS testing is given in Table 3. The receiver operating characteristic curves for the diagnosis of DSP using timed VBS and JPS testing at the great toe is given in Figure 1. The area under the curve for JPS and VBS testing at great toes was 0.63 and 0.73, respectively. The optimal cutoff value for VBS testing at the great toe to make a diagnosis of DSP was a response lasting <8 s. At this cutoff value, the sensitivity was 85% and specificity was 42.8%. The optimal cutoff point for JPS testing at the great toe was 2 or more incorrect responses. At this cutoff, the sensitivity was 33% and specificity was 87.6%.
| Age | Vibration sense great toe right | Vibration sense great toe left | Vibration sense medial malleolus right | Vibration sense medial malleolus left | Vibration sense tibial tuberosity right | Vibration sense tibial tuberosity left | Vibration sense thumb right | Vibration sense thumb left |
|---|---|---|---|---|---|---|---|---|
| 18-29, mean±SD | 8.7±1.9 | 8.8±2.2 | 7.5±1.9 | 7.8±1.94 | 6.3±1.5 | 6.5±1.8 | 11.0±2.3 | 11.5±2.3 |
| 30-39, mean±SD | 8.1±2.1 | 8.0±2.2 | 7.3±2.4 | 7.4±2.3 | 6.5±2.2 | 6.3±2.2 | 10.9±3.0 | 11.1±2.9 |
| 40-49, mean±SD | 8.1±2.0 | 8.0±2.1 | 7.23±2.1 | 7.0±1.8 | 6.9±2.0 | 6.5±1.9 | 10.4±2.3 | 11.2±2.9 |
| 50-59, mean±SD | 6.9±2.5 | 7.6±2.9 | 6.9±2.82 | 7.2±2.8 | 7.3±3.2 | 6.4±2.2 | 9.9±2.0 | 9.9±2.0 |
| 60-69, mean±SD | 7.1±3.4 | 6.3±3.0 | 6.6±1.8 | 5.8±1.5 | 6.8±2.2 | 6.0±2.6 | 10.3±2.3 | 10.5±2.1 |
| Total, mean±SD | 8.0±2.3 | 8.1±2.4 | 7.2±2.3 | 7.3±2.3 | 6.7±2.3 | 6.4±2.0 | 10.6±2.5 | 10.9±2.6 |
SD: Standard deviation
| Patients with DSP | Normal control | |
|---|---|---|
| n | 127 | 194 |
| Age (mean±SD) | 48.7±14.5 | 39.7±14.5 |
| Gender | ||
| Male | 77 | 112 |
| Female | 50 | 82 |
| Etiology of DSP (%) | ||
| Diabetes | 49 (38.6) | - |
| CMT | 7 (5.5) | - |
| Paraproteinemia | 6 (4.7) | - |
| CIDP | 12 (9.5) | - |
| Other chronic immune mediated | 17 (13.39) | - |
| Hansen’s disease | 3 (2.4) | - |
| Cancer chemotherapy related | 2 (1.6) | - |
| Cryptogenic etiology | 37 (29.13) | - |
| Sural SNAPs (µV) | 10.8 (1.06) | 29.8 (0.63) |
| Superficial peroneal SNAPs (µV) | 8.7 (0.90) | 28.8 (0.73) |
SD: Standard deviation, DSP: Distal sensory polyneuropathy, CMT: Charcot–Marie–Tooth disease. CIDP: Chronic inflammatory demyelinating polyneuropathy, SNAPs: Sensory nerve action potentials
| Test | Area under ROC | Optimal cutoff value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| Joint position sense at the big toe | 0.63 | 2 or more incorrect trials | 33.0 | 87.6 |
| Vibration sense at the big toe | 0.73 | <8 s at the great toe | 85.0 | 42.8 |
ROC: Receiver operator characteristic curve
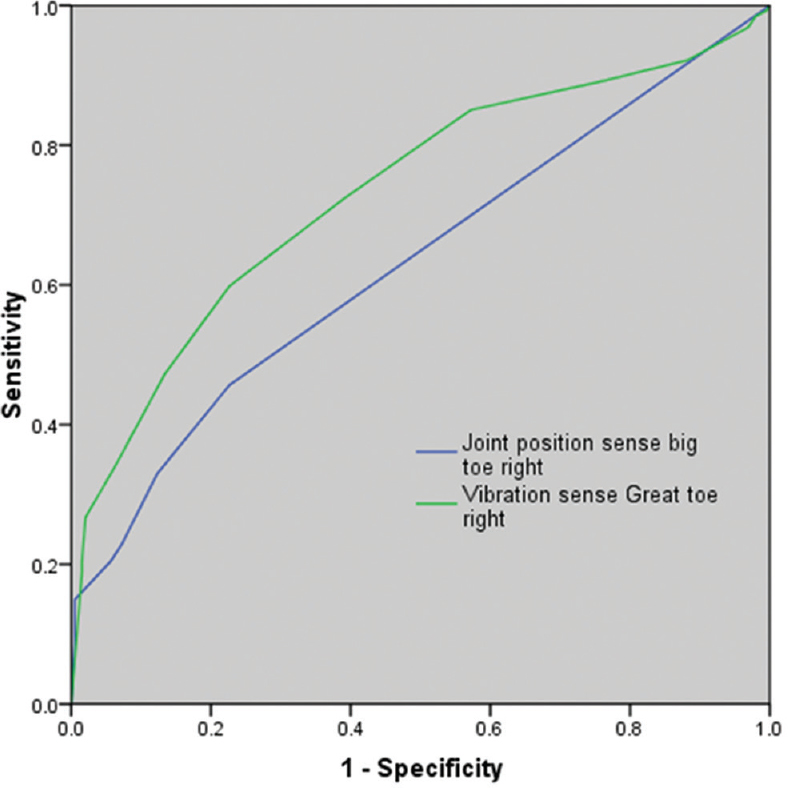
- Receiver operator characteristic curves for the diagnosis of distal sensory polyneuropathy using joint position sense and vibration sense testing at the great toe. Receiver operating characteristic curves for the diagnosis of distal sensory polyneuropathy using vibration sense and joint position sense at the great toe. The area under the curve for joint position sense and vibration sense at the great toes is 0.63 and 0.73, respectively. The optimal cutoff value for vibration sense testing at the great toe to make the diagnosis of distal sensory polyneuropathy was a response lasting <8 s. At this cutoff value, the sensitivity was 85% and specificity was 42.8%. The optimal cutoff point for joint position sense testing at the great toe was 2 or more incorrect responses. At this cutoff, the sensitivity was 33% and specificity was 87.6%
DISCUSSION
Our study shows a high sensitivity for VBS testing and a high specificity for joint position testing in the diagnosis of DSP. The etiology of DSP in our patient group was varied with diabetes mellitus being the most common cause. The role of sensory examination has already been studied in diabetics with DSP and found to be useful.[789] The second most common etiology for DSP was cryptogenic/idiopathic DSP. Previous studies on DSP have shown a similar proportion of cryptogenic DSP.[121] Hence, our study results could be applied to DSP of varied etiology, especially diabetes mellitus and cryptogenic DSP. In a study similar to ours, Abraham et al. have found a higher sensitivity and specificity for neurological examination in the diagnosis of DSP.[12] The differences in the results could be related to the differences in study setting, severity of DSP, and methods of testing. Our study was prospective, examiners were blinded to the clinical history and electrophysiological data, and also, only large fiber sensations were tested. However, the study by Abraham et al. was a retrospective study where the examiners were not blinded to the patient's clinical history, reason for referral, and suspected diagnosis. Overestimation of neurological signs is known in this setting and could be a possible contributing factor.[7] JPS testing at the great toe has been used for assessing the proprioceptive pathways.[14] Detection of movements at the interphalangeal joint of the great toe is known to be markedly worse than that of other joints, and a minimum displacement of at least 20° is needed to perceive the direction of movement.[22] Furthermore, as DSP affects the longest nerves of the lower limbs first, testing at the great toes for JPS is ideal for testing for DSP. In our study, JPS was found to be highly specific (87.6%) when there were 2 or more errors in a trial of 10. Hence, JPS can be used to rule in the diagnosis of DSP when further testing is not possible. Bedside tuning fork tests, using a 128-Hz tuning fork for assessing VBS can be qualitative or quantitative. In one method of qualitative testing, complete loss of the vibration sensation is categorized as abnormal. Other qualitative methods of testing include comparing the patient's perception with that of the examiner. This is done by placing the examiner's finger on the opposite surface of the joint being tested from the tuning fork, and noting if the vibration persists after the patient no longer senses it, or assessing the patient's threshold against the examiners, by applying the tuning fork to their finger.[23] The quantitative method involves noting the duration of perceived vibration using a stopwatch. In order to standardize the stimulus, the examiner must strike the 128-Hz tuning fork against the palm of the free hand just hard enough to make the metal ends meet to produce a metallic clanging sound. A similar method has been previously described by in Kurtzke in the Expanded Disability Status Scale for testing sensations in patients with multiple sclerosis, and a cutoff of 10 s was suggested.[24] In a study by Oyer et al., the mean duration of perceived vibration was 10.2 s, and <8 s was taken as abnormal.[11] However, there was no comparison done with electrophysiological testing. In their study, the timed VBS of <4 s correlated with the results of the monofilaments and the risk of developing a foot ulcer.[11] In our study, the cutoff value of 8 s for timed VBS had a high sensitivity (85.0%) and hence can be used to rule out DSP. Based on our results we suggest, timed VBS testing and JPS testing are useful tools to evaluate for DSP in the setting of the neurology office, community screening, and in other resource-poor settings where further electrophysiological testing is not available. The limitations of our study include a tertiary care hospital-based setting, limited number of elderly patients and controls, and lack of data on the severity of DSP.
CONCLUSION
Neurological examination continues to have an important role in the diagnosis of DSP. For timed VBS, a duration of >8 s at the great toe was a useful test to rule out DSP, and JPS testing at the great toe, 2 or more errors in a trial of 10, is a useful test in ruling in the diagnosis of DSP.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Epidemiology of peripheral neuropathy. J Neurol Neurosurg Psychiatry. 1997;62:310-8.
- [Google Scholar]
- Diagnostic and Therapeutic Advances: Distal symmetric polyneuropathy: A review. JAMA. 2015;314:2172-81.
- [Google Scholar]
- Distal symmetric polyneuropathy: A definition for clinical research: Report of the American Academy of Neurology, the American Association of Electrodiagnostic medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2005;64:199-207.
- [Google Scholar]
- Peripheral neuropathy in prediabetes and the metabolic syndrome. J Diabetes Investig. 2017;8:646-55.
- [Google Scholar]
- The diagnostic criteria for small fibre neuropathy: From symptoms to neuropathology. Brain. 2008;131:1912-25.
- [Google Scholar]
- The beautiful and ethereal neurological exam: An appeal for research. Ann Neurol. 2011;70:A9-10.
- [Google Scholar]
- Signs and symptoms versus nerve conduction studies to diagnose diabetic sensorimotor polyneuropathy: Cl vs. NPhys trial. Muscle Nerve. 2010;42:157-64.
- [Google Scholar]
- Back to basics in diagnosing diabetic polyneuropathy with the tuning fork! Diabetes Care. 2005;28:2201-5.
- [Google Scholar]
- Sensitivity and specificity of a new indicator test (Neuropad) for the diagnosis of peripheral neuropathy in type 2 diabetes patients: A comparison with clinical examination and nerve conduction study. J Diabetes Complications. 2007;21:353-8.
- [Google Scholar]
- Assessment of vibratory sensation with a tuning fork at different sites in Japanese patients with diabetes mellitus. J Diabetes Investig. 2014;5:90-3.
- [Google Scholar]
- Quantitative assessment of diabetic peripheral neuropathy with use of the clanging tuning fork test. Endocr Pract. 2007;13:5-10.
- [Google Scholar]
- The sensitivity and specificity of the neurological examination in polyneuropathy patients with clinical and electrophysiological correlations. PLoS One. 2017;12:e0171597.
- [Google Scholar]
- Joint position sense and vibration sense: Anatomical organisation and assessment. J Neurol Neurosurg Psychiatry. 2002;73:473-7.
- [Google Scholar]
- Quantitative clinical electrophysiology in the evaluation of nerve injury and regeneration. Muscle Nerve. 1990;13:822-8.
- [Google Scholar]
- Electromyography and neuromuscular disorders: Clinical electrophysiologic correlations. McGill J Med MJM. 2006;9:173.
- [Google Scholar]
- Electrodiagnostic approach to the patient with suspected mononeuropathy of the lower extremity. Neurol Clin. 2002;20:479-501, vii.
- [Google Scholar]
- A new method of superficial peroneal nerve conduction studies. Electromyogr Clin Neurophysiol. 2003;43:507-10.
- [Google Scholar]
- Intensive evaluation of referred unclassified neuropathies yields improved diagnosis. Ann Neurol. 1981;10:222-6.
- [Google Scholar]
- Diagnostic accuracy of qualitative versus quantitative tuning forks: Outcome measure for neuropathy. J Clin Neuromuscul Dis. 2014;15:96-101.
- [Google Scholar]
- Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS) Neurology. 1983;33:1444-52.
- [Google Scholar]






