Translate this page into:
Mindbomb Homolog-1 Index in the Prognosis of High-Grade Glioma and Its Clinicopathological Correlation
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Gliomas are the most common brain tumors in adults originating from the glial cells. Glioblastoma multiforme is the most malignant and frequent among all gliomas. In recent years, the antibody Mindbomb Homolog-1 (MIB-1) has evolved as a measure of the proliferative nature of the glial tumors. This study aims to investigate the MIB-1 index value as an independent prognostic factor in high-grade gliomas and its correlation with outcome and survival.
Materials and Methods:
Mean MIB-1 index was determined in 51 high-grade glioma tissue samples in formalin. Its correlation with outcome by assessing the clinicoradiological parameters and median survival of patients in months were assessed. Survival analysis was studied by using the Kaplan–Meier bivariate analysis and Cox proportional ratio.
Results:
Preoperative Karnofsky Performance Score, WHO-PS, Neurological Performance Scale, and Mini–Mental Status Examination (MMSE) were statistically significant with respect to outcome and survival, whereas tumor factors such as size and perilesional edema were not. In particular, midline-crossing tumors and deep-seated tumors were significantly associated with high MIB-1 index and by correlation with outcome. There were significantly higher number (P < 0.0001) of patients with Grade IV tumors, with an MIB-1 index value above an arbitrary cutoff of 10% compared to Grade III tumors. In addition, median survival period of patients with low MIB-1 index was longer irrespective of tumor grade.
Conclusion:
Significant correlation between high-grade glioma and MIB-1 index suggests MIB-1 index to be a good prognostic tool, with MIB-1 index and midline-crossing variables being independent prognostic parameters.
Keywords
Brain tumor
high-grade glioma
Ki-67
Mindbomb Homolog-1 index
midline-crossing tumors
tumor prognostication
INTRODUCTION
Glial tumors constitute 30% of central nervous system tumors including the brain and spine and 80% of all malignant brain tumors.[1] Patients with high-grade gliomas (Grade III and IV) show, despite them being an aggressive group of tumors, a wide range of survivability and differential response to treatment protocols.[1]
A number of markers that correlate with the nature of these tumors also correlate to survival and outcomes. Among these, Ki-67 is a widely available and employed marker which is routinely used to quantify proliferative nature recognized by Mindbomb Homolog-1 (MIB-1) antibody.[234] This marker has also been studied to be correlated with survival and thus serve as an adjuvant to prognosticate high-grade gliomas.
At our institution, we found a similar wide variability in survivability among patients with high-grade gliomas ranging from 2 months to 86 months. Due to a relative dearth of material evaluating MIB-1 index and its relation to survival and tumor factors specifically in high-grade gliomas, this study was undertaken to evaluate these correlations.
MATERIALS AND METHODS
Patients who were surgically treated for high-grade gliomas at a primary institution between May 2013 and May 2017 were prospectively studied. The study was done in collaboration with the department of neuropathology, at a secondary institution.
Inclusion criteria
All patients who had histologically proven high-grade glioma (Grade III and Grade IV) according to the WHO criteria and were treated surgically by biopsy Stereotactic Biopsy (STB), subtotal excision, and gross total resection were considered. This included patients with anaplastic astrocytoma, anaplastic oligodendroglioma, anaplastic oligoastrocytoma, anaplastic ependymoma (Grade III) and glioblastoma multiforme, gliosarcoma, astroblastoma (Grade IV) as well as patients with previously treated low-grade gliomas who showed recurrence to high-grade glioma. All patients included in the study were followed up for more than 1 year. However, all cases of mortality within 1 year of surgery were also included in the study.
After surgical intervention, the patients were referred to oncology team for further chemotherapy/radiotherapy.
Exclusion criteria
Any patient who had either histologically proven low-grade glioma (Grade I and Grade II) or had inconclusive histopathology, who were not managed surgically, had brainstem gliomas, underwent preoperative cranial irradiation, those with <1-year follow-up, or those lost to follow-up were excluded from the study.[5]
Parameters studied
The parameters that were studied included patients’ demographics such as age and sex; duration of follow-up after surgery; various imaging parameters including tumor size, location of tumor (superficial/deep), midline-crossing tumor, peritumoral edema, extent of resection; and tumor residue on postoperative scan. Other parameters that were studied were pathology findings and MIB-1 index, extent of resection, chemoradiation status, recurrence status, and survival in months. Karnofsky Performance Status (KPS), Eastern Cooperative Oncology Group/WHO-PS, Neurological Performance Scale (NPS), Mini–Mental Scoring Examination (MMSE), and PIGNATTI index were evaluated for each patient.
Figure 1 shows the magnetic resonance imaging axial scans of three patients with high-grade glioma. Figures 2 and 3 show the photomicrographs of histological sections of glioblastoma, anaplastic oligodendroglioma, and anaplastic astrocytoma and with proliferation index (MIB-1).
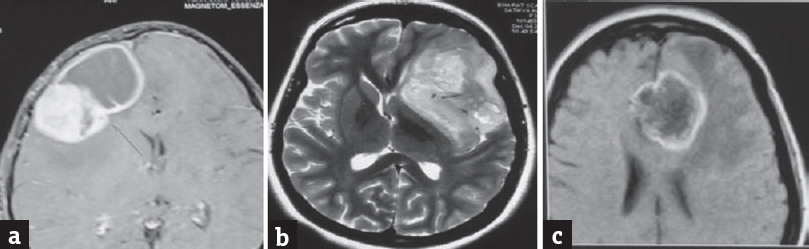
- (a) MRI axial contrast of an 8-year-old boy showing large solid cystic right frontal lesion with intense heterogeneous enhancement of solid and thick rim enhancement of cystic component. HPE was GBM. (b) MRI T2 axial contrast of a 33-year-old female showing large ill-defined T2-weighted hyperintense left insular lesion with few intralesional cystic areas and no significant contrast enhancement. HPE was anaplastic oligoastrocytoma. (c) MRI axial contrast of a 61-year-old female showing a thick-walled peripheral enhancing anterior corpus callosum midline-crossing lesion with central necrosis. HPE was GBM. MRI: Magnetic resonance imaging, GBM: Glioblastoma multiforme, HPE: Histopathological examination

- Photomicrographs of a glioblastoma composed of pleomorphic astrocytes exhibiting nuclear atypia and mitosis (a, arrow) with microvascular proliferation (b, arrow) and necrosis (c). Proliferation index is high (d, MIB-1). MIB-1: Mindbomb Homolog-1

- Photomicrographs of an anaplastic oligodendroglioma (a and b) characterized by clear cells arranged in sheets separated by chicken-wire vasculature (a) and exhibiting high proliferation (b, MIB-1); an anaplastic astrocytoma (c and d) composed of fibrillary, gemistocytic astrocytes with increased mitosis (c, arrow) and proliferation (d, MIB-1). MIB-1: Mindbomb Homolog-1
Follow-up
The patients were assessed clinically and radiologically for every 3 months until 1 year and annual follow-up thereafter. Imaging data for recurrence, patient factors, and survival were evaluated. For the purpose of this study, the survival period was calculated from the date of diagnosis to mortality or until the last follow-up.
IMMUNOHISTOCHEMICAL STAINING FOR KI-67: PROCEDURE OF IMMUNOHISTOCHEMISTRY
Immunohistochemistry was performed using the Ventana BenchMark automated staining system Ventana BenchMark-XT, Ventana Medical Systems, USA. on 4-μm tissue microarray sections using MIB-1 antibody (clone Ki-67, Dako, 1:200). Briefly, the slides were deparaffinized using xylene followed by antigen retrieval and incubation with primary antibody followed by incubation with secondary antibody. 3,3’-diaminobenzidine was used as the chromogenic substrate. Slides were treated with hematoxylin, dried, and mounted with DPX. As negative controls, primary antibody was replaced by phosphate-buffered saline and processed as above. Positive controls were run with each batch.
EVALUATION OF MINDBOMB HOMOLOG-1 INDEX IMMUNOSTAINING
Each slide was evaluated through ten random high-power fields (×40), which include counting the number of positive and negative nuclei. Every brown-stained nucleus is considered positive irrespective of intensity. The number of positive nuclei was divided by the total estimated number of counted nuclei, to calculate proliferative or MIB-1 index for each case. Necrotic or thick areas and severely overlapping tumor cells were avoided during the tumor evaluation.
Statistical significance of various patient factors and tumor factors with respect to patients’ outcome and survival was calculated. The analyses were done using SAS software tool, SAS 9.4 (SAS Institute, Cary, North Carolina, USA). t-test and other tests for correlation analysis such as Fisher's test were run as required. P =0.05 was considered statistically significant. Both univariate and multivariate analyses were done using Cox proportional method, and the respective survival curves were estimated using Kaplan–Meier nonparametric method/log rank tests.
RESULTS
A total of 205 patients were treated for glioma at a primary institution from May 2013 to May 2017. Of these, 105 patients were Grade I, Grade II, or brainstem gliomas and were excluded from the study. Of the remaining 110 patients, 51 patients with high-grade glioma met the inclusion/exclusion criteria and were considered in this study; of these, there were 20 and 31 patients in Grade III and Grade IV, respectively [Figure 4]. Among Grade III tumors, five were Grade II progressing to Grade III, six were anaplastic astrocytoma, four were anaplastic oligodendroglioma, and five were anaplastic oligoastrocytoma. All the 31 patients of Grade IV glioma were glioblastoma multiforme (GBM).

- Flowchart of patients who met inclusion/exclusion criteria for this study
The mean age of the patients was 46.45 ± 16.13 years, with the highest number of patients (33%) being in the 40–60 years’ age group. There were 29 males and 22 females, resulting in a male-to-female ratio of 1.3:1. Frontal lobe was most commonly involved (18 cases – 13 cases being only frontal lobe and 5 being a combination of frontal with other lobes). There were 17 cases of midline-crossing tumors.
All patients had perilesional edema, with Grade I, Grade II, and Grade III perilesional edema being present in 33.33% (17), 62.75% (32), and 3.92% (2) of patients, respectively.
Gross total resection of lesion defined as resection of >95% of tumor volume was performed for 45 patients (88.24%), whereas subtotal and STB cases were three (5.88%) each.
Recurrence in our study was defined as either (i) persistence of no lesion in repeat scan in cases, which were free of lesion in postoperative scan, or (ii) no increase in the size of residual lesion if it was present in the postoperative scan. In our study, 23 patients were adjudged to have no recurrence. Among these, residual lesion was not seen in postoperative scans for 17 patients, whereas there were six patients in whom residual lesion did not increase in size. On the other hand, 28 patients imaging showed increase in residual size or reappearance of lesion and were adjudged to have recurrence.
Of the 23 patients who were adjudged to have no recurrence, 15 patients were alive, whereas of the 28 patients with recurrence, only 4 were alive at the end of the study.
Mean MIB-1 index of the study group was found to be 20 ± 13. Mean MIB-1 indexes of Grade III and Grade IV tumors were calculated as 11.2 ± 9 and 26 ± 11.8, respectively. MIB-1 index was grouped as low (≤10) and high (>10). There was statistically significant difference (P = 0.0001) in the distribution of MIB-1 index across Grade III and IV tumors, with Grade III tumors predominantly having low MIB-1 index and Grade IV tumors having predominantly high MIB-1 index [Table 1].
| Parameters | MIB-1 index | P | Significance | |
|---|---|---|---|---|
| Low (≤10) | High (>10) | |||
| Tumor factors | ||||
| Tumor size (mm) | ||||
| ≤30 | 1 | 2 | 0.503 | Statistically not significant (P<0.5) |
| 31-40 | 4 | 5 | ||
| 41-50 | 8 | 11 | ||
| 51-60 | 3 | 8 | ||
| >60 | 1 | 8 | ||
| Perilesional edema | ||||
| Grade I | 8 | 9 | 0.177 | Statistically not significant (P<0.5) |
| Grade II | 8 | 24 | ||
| Grade III | 1 | 2 | ||
| Midline-crossing tumor | ||||
| Yes | 2 | 15 | 0.021 | Statistically significant (P<0.05) |
| No | 15 | 19 | ||
| Tumor grade | ||||
| Grade III | 14 | 6 | 0.0001 | Extremely statistically significant (P<0.0005) |
| Grade IV | 3 | 28 | ||
| Outcome | ||||
| Alive | 14 | 5 | 0.0001 | Extremely statistically significant (P<0.0005) |
| Died | 3 | 29 | ||
MIB-1: Mindbomb Homolog-1
Midline-crossing tumors were also found to be significantly correlated with high MIB-1 index (P = 0.021).
Other tumor factors such as tumor size and perilesional edema did not have statistically significant correlation with MIB-1 index grouping.
Grade III glioma patients had a median survival of 18 months; among these, patients with low MIB-1 index had longer median survival (35 months) as compared to high MIB-1 index (3.5 months). Grade IV patients had median survival of 5.5 months; among these, patients with low index value had longer median survival (12 months) compared to high MIB-1 index of Grade IV gliomas (5 months). These differences in median survival between low and high MIB-1 index in either grade were statistically significant (P < 0.001) [Table 2].
| MIB-1 index | Alive | Died | Median survival (months) | Significance |
|---|---|---|---|---|
| Grade 3 | ||||
| Total | 11 | 9 | 18 | |
| Low (≤10) | 11 | 3 | 35 | P=0.0021 |
| High (>10) | 0 | 6 | 3.5 | Highly significant |
| Grade 4 | ||||
| Total | 8 | 23 | 5.5 | |
| Low (≤10) | 3 | 0 | 12 | P=0.0125 |
| High (>10) | 5 | 23 | 5 | Highly significant |
MIB-1: Mindbomb Homolog-1
Survival analysis curves for Grade III and Grade IV glioma were obtained using log rank test for Kaplan–Meier method and two-tailed P test. The results showed that the survival rate of Grade III and Grade IV gliomas was statistically significant in relation to MIB-1 index with P = 0.00025 and 0.04, respectively [Figure 5].

- Kaplan–Meier estimates of Grade III and Grade IV survival functions
Patient outcome was not statistically significant in relation to tumor factors such as tumor size, eloquent cortex, and perilesional edema. However, midline-crossing tumors showed significant correlation with the outcome (P = 0.0128). Clinical parameters such as KPS score, WHO-PS, NPS scale, PIGNATTI index, and MMSE were found to be significantly correlated with outcome; among these, preoperative and postoperative KPS score showed extremely significant correlation with respect to survival and outcome (P < 0. 005) [Table 3].
| Tumor factors | Outcome | P | Significance | |
|---|---|---|---|---|
| Alive | Died | |||
| Tumor size (mm) | ||||
| ≤ s | 1 | 2 | 0.467 | Statistically not significant (P<0.5) |
| 31-40 | 4 | 5 | ||
| 41-50 | 8 | 11 | ||
| 51-60 | 5 | 6 | ||
| >60 | 1 | 8 | ||
| Eloquent cortex | ||||
| No | 16 | 24 | 0.50 | Not statistically significant (P<0.5) |
| Yes | 3 | 8 | ||
| Deep-seated tumor | ||||
| No | 19 | 30 | 0.5321 | Statistically not significant (P<0.5) |
| Yes | 0 | 2 | ||
| Midline-crossing tumor | ||||
| No | 17 | 17 | 0.0128 | Statistically significant (P<0.05) |
| Yes | 2 | 15 | ||
| Tumor status | ||||
| No recurrence | 14 | 3 | 0.001 | Highly statistically significant (P<0.005) |
| Recurrence | 4 | 24 | ||
| Residue | 1 | 5 | ||
| Postsurgery chemoradiation status | ||||
| Not given | 0 | 10 | 0.013 | Statistically significant (P<0.05) |
| Only chemotherapy | 0 | 0 | ||
| Only radiation | 3 | 5 | ||
| Chemo radiation | 16 | 17 | ||
| Glioma | ||||
| Grade III | 14 | 3 | 0.0001 | Extremely statistically significant (P<0.0005) |
| Grade IV | 5 | 29 | ||
| Grade III | ||||
| Low (≤10) | 11 | 3 | 0.0021 | Highly statistically significant (P<0.005) |
| High (>10) | 0 | 6 | ||
| Grade IV | ||||
| Low (≤10) | 3 | 0 | 0.012 | Statistically significant (P<0.05) |
| High (>10) | 5 | 23 | ||
Survival rate of patients who completed chemoradiation has 13-month median survival rate compared to 2.5 months who did not. In addition, chemoradiation was significantly correlated with the recurrence of tumor postsurgery (P = 0.001).
All the parameters were subjected to multivariate analysis using Cox proportional method. Hazard ratio, 95% confidence level, and P value of the predominant tumor and patient parameters were calculated [Table 4]. In multivariate analysis, deep-seatedness of tumor was found to be significant, in reversal of the result from univariate analysis. MIB-1 index and midline-crossing tumor continued to remain highly significant in multivariate analysis, and thus were found to be significant independent prognostic markers.
| Factors | Hazard ratio | 95% confidence level | Regression coefficient | P | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Tumor grade | 0.4104 | 0.1305 | 1.2910 | −0.8906 | 0.1277 |
| MIB index | 16.5864 | 3.4044 | 80.8105 | 2.8086 | 0.0005 |
| Midline-crossing tumor | 4.2988 | 1.6100 | 11.477 | 1.4583 | 0.0036 |
| Pre-KPS | 1.3495 | 0.7542 | 5.0388 | 0.9676 | 0.0368 |
| Post-KPS | 2.2061 | 0.8925 | 5.4229 | 0.7912 | 0.0846 |
| Deep seated | 25.68 | 2.618 | 36.67 | 3.2458 | 0.0124 |
| Edema | 1.8107 | 1.5214 | 4.0120 | 0.2170 | 0.6429 |
| Eloquent cortex | 0.731 | 0.2614 | 2.0508 | −0.3118 | 0.5530 |
| Extent of resection | 0.5622 | 0.2461 | 1.2841 | −0.5759 | 0.1717 |
KPS: Karnofsky Performance score, MIB: Mindbomb Homolog-1
In a nutshell, MIB-1 index showed extremely statistically significant correlation (P < 0.0005) and was found to be a strong independent prognostic measure for outcome in high-grade glioma.
DISCUSSION
Gliomas are the most common brain tumors in adults and constitute up to almost 80% of all malignant brain tumors and among these high-grade gliomas (Grade III and Grade IV astrocytomas in the WHO classification) constitute 65% of all brain tumors.[1] Prognosis of these tumors varies even within each individual histological grade despite equivalent treatment. Among high-grade gliomas, although survival and outcome of Grade III gliomas are significantly better than Grade IV gliomas, there is a high survival variability even within each of these two grades.[6]
Several studies have correlated clinical and patient data (age, KPS, clinical symptoms, and duration), tumor factors (including extent, location, edema, histopathological grade, MIB index, IDH 1 and 2, and 1p/19q co-deletion), and treatment modalities (extent of surgical resection, radiation, and adjuvant chemotherapy) with survival.[789]
Proliferative potentials of gliomas provide direct insight about their biological behavior and hence into the prognosis of patients.[10] Proliferative indices vary significantly within similar histological grades with significant overlap among various grades. Tumor proliferation can be assessed using a number of markers including DNA polymerase α, p105, DNA topoisomerase II-α, PCNA, and Ki-67.[11] These proliferative markers estimate the growth of neoplasm and hence aid in prognostication.[11] Several studies have found Ki-67 to be a useful biological marker for evaluating proliferative index and hence for prognosticating survival and outcome.[11]
Ki-67 was discovered by Gerdes et al. in 1983 by immunizing mice with nuclei of the Hodgkin lymphoma cell line L428.[2] Antigen Ki-67, also known as Ki-67 or MKI67, is a protein that is encoded by the MKI67 gene (antigen identified by monoclonal antibody Ki-67) in humans.[212] The antigen is expressed in all phases of the cell cycle except for G0 and the early parts of G1, although the precise function of the Ki-67 protein is still unclear.[34] Initially, the Ki-67 antibody could only be used on fresh or frozen tissue, as fixation greatly reduced the immunostaining. The discovery of MIB-1 antibody, however, enabled the detection of Ki-67 antigen in formalin-fixed and paraffin-embedded tissue sections, thus greatly improving the value of the detection of Ki-67 antigen.
Our key interest was to analyze MIB index with reference to both intra- and intergrade survival variability among high-grade gliomas. MIB index was chosen as a proliferative index due to its wide availability, familiarity, and cost-effectiveness.
Different studies have reported varying cutoffs for MIB index to help define prognostication in gliomas. Enestrom et al. proposed a cutoff point as 10%.[5] Other authors like Sallien et al. employed a cutoff of 15.3%, Di et al. of 8.0%, Hsu et al. of 1.5%, McKeever et al. of 2.5%, Schiffer et al. of 8.0%, and Jaros et al. of 5%, whereas Ellison et al. suggested a value of 2.[13141516171819] These variations are due to interlaboratory technical issues such as standardization, staining, antigen retrieval methods, individual variability, dilution techniques, fixation protocols, and incubation time.[1520] In our study, a cutoff point of 10% was proposed for the prognostic measure of high-grade gliomas which showed extremely statistically significant P = 0.0001 [Table 5]. In our study, we found a significant correlation between MIB index and Grade III and Grade IV gliomas. Grade III tumors predominantly had a low MIB index, whereas Grade IV tumors had a high MIB index. This was similar to the conclusions by Wakimoto et al., Thotakura et al., and Rathi et al.[71021] However, this was contested in other studies by Hsu et al., Johannessen and Torp, and Rodríguez-Pereira et al.[152022]
| Study | Anaplastic astrocytomas | Glioblastomas |
|---|---|---|
| Wakimoto et al., 1996 (72 cases) | 18.4 | 31.6 |
| Hsu et al., 1997 (80 cases) | 8.75 (0-32.43) | 9.12 (0-29.83) |
| Giannini et al., 1999 (271 cases) | 6 (0.1-25.7) | 9.1 (0.3-36) |
| Rodriguez-pereria et al., 2000 (137 cases) | 34.5 (16-51.6) | 46 (3.8-63) |
| Tihan et al., 2000 (50 cases) | 11.4 | 20.2 |
| Ralte et al., 2001 (64 initial tumors) | 9.65 (0.5-19.6) | 10.33 (0.4-23.5) |
| Rathi et al., 2007 (90 cases) | 8.74 (2.5-26) | 20.54 (5-45.2) |
| Ambroise et al., 2011 (145 cases) | 7.45 (0.5-22.0) | 13.85 (1.2-59.0) |
| Ambroise et al., 2012 (105 cases) | 28.24 (16-34.8) | 38.7 (20-52) |
| Present study 2017 (51 cases) | 11.2±9 | 26±11.8 |
Irrespective of the histological grade, we found low MIB-1 index (≤10) to be correlated with longer survival among high-grade gliomas. In addition, midline-crossing tumors were found to be associated with high MIB-1 index values. This correlation has not been reported in literature to the best of our knowledge.
Other factors that were considered to have a bearing on the outcome of patients were age; sex; clinical scores such as KPS, WHO-PS, NPS; perilesional edema; location of tumor; and extent of resection. Age of the patients ranged from 3 years to 72 years, with the largest number of patients in the age group of 40–60 years (33%). The range of age was in similarity to studies conducted by Thotakura et al., Bloom et al., and Jaskolsky et al.[102324] Our study group had a male predominance similar to Thotakura et al. and Ganju et al.[1025] Multivariate analysis showed that the age and sex did not have significant correlation with patients’ outcome. This was in concurrence with Ambroise et al., in whose study age, though correlated with outcomes in univariate analysis, lost significance in multivariate analysis.[26] There was no lobar predilection for GBM in our study. This was in contrast to Simpson et al. who had observed 43% of GBM in frontal lobe.[27] However, it may be noted that patients in our study had frontal lobe predominance if we consider high-grade glioma as a whole.
Outcome and survival in our study showed a significant correlation to preoperative and postoperative parameters such as KPS, WHO-PS, NPS scale, and MMSE score, of which the correlation was highly significant with KPS. In fact, good preoperative and postoperative KPS have consistently been shown to be correlated with better outcomes in a number of studies. Pierallini et al., for preoperative KPS, and Stark et al., for preoperative and postoperative KPS in their series, showed a similar correlations.[2829] Wakimoto et al. and Lacroir et al. also showed similar results.[730] Hammoud et al., in their study, concluded that KPS above 70 carries a better outcome and survival. In our study, we achieved similar significantly higher survival and better outcomes if KPS was above 80.[31]
Perilesional edema has been reported by a number of authors to be significantly related to outcomes. The conclusions, however, are not unanimous. While Whitney et al. found lesser grades of edema to have better outcomes,[32] Hammoud et al. found that Grade I and III edema have better outcomes than Grade II.[31] In our study, however, we found no significant correlation between grades of edema and outcomes.
Extent of resection is another factor that has universally been found related to outcomes. A number of studies by Pierallini et al., Hess et al., and Stark et al. have concluded the extent of resection to be favorably related to outcomes.[282932] In our study, however, the correlation between resection and outcomes was not significant. This may be due to the smaller sample size and comparatively shorter duration of follow-up.
Location of tumor has been variously correlated to outcomes in different studies. Whereas Stark et al. found temporal gliomas to be a better prognostic factor, Pigott et al. found the same results for frontal gliomas.[2933] In contrast, Gehan et al. found parietal gliomas to have a worse prognosis.[34] On the other hand, a number of other authors like Hammoud et al. and Byar et al. found no significant difference in outcomes between gliomas at different locations.[3335] In our study, overall location was not significantly related to outcomes.
If we consider tumor location as superficially located and deeply located tumor, we found no significant relation between outcomes and location on univariate analysis. However, similar to Wakimoto et al., the deep-seatedness of tumor gained significance on multivariate analysis.[7]
An important point of note was relation of outcome to midline-crossing tumors. As noted before, midline-crossing tumors were associated with high MIB index. However, multivariate analysis showed midline-crossing tumors to be an independent prognostic marker with statistically significant correlation. An extensive search of published literature showed no prior study, wherein this correlation between midline-crossing tumor and outcome has been evaluated.
CONCLUSION
We found MIB index to be a good prognostic indicator for high-grade glioma. MIB-1 index grouping for Grade III and Grade IV glioma as low (≤10) and high (>10) showed a strong correlation with respect to outcome and survival. Other parameters that strongly influenced outcome and survival were KPS and location of tumor – deep-seated and midline-crossing tumors.
MIB index is suited as a viable prognostic tool especially in resource-limited settings or in environments where advanced testing may not be available due to its wide familiarity among pathologists, ease of availability, and cost-effectiveness.
Although our study provides a strong evidence of association between MIB index and high-grade glioma survival outcomes, a larger cohort study would give more strength to this conclusion.
Informed consent
Informed consents were obtained from all individual participants included in the study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31:13-20.
- [Google Scholar]
- The Ki-67 protein: From the known and the unknown. J Cell Physiol. 2000;182:311-22.
- [Google Scholar]
- Assignment of the gene (s) involved in the expression of the proliferation-related Ki-67 antigen to human chromosome 10. Hum Genet. 1989;83:297-9.
- [Google Scholar]
- Ki-67 antigen expression as a prognostic factor in primary and recurrent astrocytomas. Neurochirurgie. 1998;44:25-30.
- [Google Scholar]
- MinADC values predict prognosis in patients with low-grade and high-grade gliomas by 3.0-T MRI. Int J Clin Exp Med. 2016;9:21490-7.
- [Google Scholar]
- Prognostic significance of Ki-67 labeling indices obtained using MIB-1 monoclonal antibody in patients with supratentorial astrocytomas. Cancer. 1996;77:373-80.
- [Google Scholar]
- Recursive partitioning analysis of prognostic factors in three radiation therapy oncology group malignant glioma trials. J Natl Cancer Inst. 1993;85:704-10.
- [Google Scholar]
- Role of Ki-67 labeling index as an adjunct to the histopathological diagnosis and grading of astrocytomas. J Cancer Res Ther. 2014;10:641-5.
- [Google Scholar]
- Evaluation of the proliferation marker Ki-67 in glioblastoma and its correlation with histopathological findings. Acad J Cancer Res. 2016;9:13-8.
- [Google Scholar]
- Ki-67 protein is associated with ribosomal RNA transcription in quiescent and proliferating cells. J Cell Physiol. 2006;206:624-35.
- [Google Scholar]
- Prognostication of astrocytoma patient survival by Ki-67 (MIB-1), PCNA, and S-phase fraction using archival paraffin-embedded samples. J Pathol. 1994;174:275-82.
- [Google Scholar]
- Proliferative potentials of glioma cells and vascular components determined with monoclonal antibody MIB-1. J Exp Clin Cancer Res. 1997;16:389-94.
- [Google Scholar]
- Use of MIB-1 (Ki-67) immunoreactivity in differentiating grade II and grade III gliomas. J Neuropathol Exp Neurol. 1997;56:857-65.
- [Google Scholar]
- MIB-1 proliferation index predicts survival among patients with grade II astrocytoma. J Neuropathol Exp Neurol. 1998;57:931-6.
- [Google Scholar]
- Proliferative activity and prognosis of low-grade astrocytomas. J Neurooncol. 1997;34:31-5.
- [Google Scholar]
- Prognostic implications of p53 protein, epidermal growth factor receptor, and Ki-67 labelling in brain tumours. Br J Cancer. 1992;66:373-85.
- [Google Scholar]
- Prognostic indicators in a range of astrocytic tumours: An immunohistochemical study with Ki-67 and p53 antibodies. J Neurol Neurosurg Psychiatry. 1995;59:413-9.
- [Google Scholar]
- The clinical value of Ki-67/MIB-1 labeling index in human astrocytomas. Pathol Oncol Res. 2006;12:143-7.
- [Google Scholar]
- Proliferative index in astrocytic tumours. Indian J Pathol Microbiol. 2007;50:754-8.
- [Google Scholar]
- Value of MIB-1 labelling index (LI) in gliomas and its correlation with other prognostic factors. A clinicopathologic study. J Neurosurg Sci. 2000;44:203-9.
- [Google Scholar]
- Low grade glioma of the cerebral hemispheres in adults: A retrospective analysis of 88 cases. Int J Radiat Oncol Biol Phys. 1990;18:783-6.
- [Google Scholar]
- Mixed gliomas. Their clinical course and results of surgery. Zentralbl Neurochir. 1987;48:120-3.
- [Google Scholar]
- Prognostic factors in gliomas. A multivariate analysis of clinical, pathologic, flow cytometric, cytogenetic, and molecular markers. Cancer. 1994;74:920-7.
- [Google Scholar]
- Practical value of MIB-1 index in predicting behavior of astrocytomas. Indian J Pathol Microbiol. 2011;54:520-5.
- [Google Scholar]
- Influence of location and extent of surgical resection on survival of patients with glioblastoma multiforme: Results of three consecutive Radiation Therapy Oncology Group (RTOG) clinical trials. Int J Radiat Oncol Biol Phys. 1993;26:239-44.
- [Google Scholar]
- Radiological assessment of necrosis in glioblastoma: Variability and prognostic value. Neuroradiology. 1998;40:150-3.
- [Google Scholar]
- Glioblastoma multiforme-report of 267 cases treated at a single institution. Surg Neurol. 2005;63:162-9.
- [Google Scholar]
- A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190-8.
- [Google Scholar]
- Prognostic significance of preoperative MRI scans in glioblastoma multiforme. J Neurooncol. 1996;27:65-73.
- [Google Scholar]
- Extent of resection as a prognostic variable in the treatment of gliomas. J Neurooncol. 1999;42:227-31.
- [Google Scholar]
- Statistical modelling in analysis of prognosis in glioblastoma multiforme: A study of clinical variables and Ki-67 index. Br J Neurosurg. 1991;5:61-6.
- [Google Scholar]
- Prognostic factors for patients with brain tumors. Natl Cancer Inst Monogr. 1977;46:189-95.
- [Google Scholar]
- Prognostic factors for malignant glioma. In: Oncology of the Nervous System. Boston, MA: Springer; 1983. p. :379-95.
- [Google Scholar]






