Translate this page into:
Regrowth of a Large Intracranial Aneurysm after On-Label Use of the Pipeline Embolization Device
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Despite the widespread use of the pipeline embolization device (PED), no complete aneurysm regrowth after its placement has been reported in the literature. We report the first case of aneurysm regrowth after the initial follow-up angiography demonstrating near-complete occlusion of the aneurysm and remodeling of the vessel with on-label PED use for a large 20 mm × 24 mm × 22 mm (width × depth × height) cavernous segment internal carotid artery (ICA) aneurysm. The patient was treated with two overlapping PED (4.5 mm × 20 mm and 5 mm × 20 mm). Follow-up angiogram at 4 months after treatment demonstrated remodeling of the ICA with a small residual component measuring approximately 7 mm × 8 mm × 7 mm. However, at 10 months after treatment, there was a complete regrowth of the aneurysm with interval growth, now measuring 25 mm × 28 mm × 18 mm. Despite the high aneurysm occlusion rates reported with the PED, persistent aneurysm filling and aneurysm regrowth, although rare, should not be overlooked.
Keywords
Aneurysm
complication
endovascular
pipeline
regrowth
INTRODUCTION
Approval of the pipeline embolization device (PED; Medtronic Inc., Minneapolis, MN, USA) in 2011 by the US Food and Drug Administration has revolutionized not only the treatment paradigm of large or giant wide-necked intracranial aneurysms in the proximal internal carotid artery (ICA) but also the treatment options available for small, distal anterior circulation, and posterior circulation aneurysms.[1] Despite the widespread use of the PED, no aneurysm regrowth after its placement has been reported in the literature, although aneurysm persistence has been noted. We report the first case of complete aneurysm regrowth after on-label PED treatment of a large cavernous segment ICA aneurysm.
CASE REPORT
This is a 76-year-old female with a medical history of hypertension, hyperlipidemia, and cardiac arrhythmia who was found to have a large 20 mm × 24 mm × 22 mm (width × depth × height) left cavernous carotid wide-necked aneurysm during a workup for gait instability in 2016. The patient was taking rivaroxaban for her cardiac arrhythmia and smoked one packet of cigarettes per day. Given the morphology and location of an aneurysm, the treatment of an aneurysm using the PED was recommended. Seven days before the procedure, the patient was started on aspirin (325 mg daily), and immediately before the procedure, her aspirin assay was therapeutic (462 aspirin reaction unit [ARU]; <551 ARU was considered therapeutic). Her rivaroxaban was withheld 48 h before her procedure.
In March 2017, the patient underwent placement of two overlapping PED (4.5 mm × 20 mm and 5 mm × 20 mm) through the Marksman microcatheter (Medtronic Inc., Minneapolis, MN, USA) and Sofia intermediate catheter (MicroVention, Aliso Viejo, CA) for the treatment of her large left cavernous carotid aneurysm [Figure 1a]. The first PED (4.5 mm × 20 mm) was deployed spanning from the supraclinoid ICA into the neck of the aneurysm. Then, the second PED (5 mm × 20 mm) spanning from the cavernous ICA, overlapping the distal PED, into the vertical segment of the petrous ICA was placed, covering the neck of an aneurysm. The entire flow-diverter stent construct spanned from the supraclinoid ICA to the vertical segment of the petrous ICA [Figure 1b]. Final postprocedural control angiogram demonstrated significant contrast stasis within the aneurysm sac [Figure 1c] while fully heparinized. The patient was continued on her aspirin and restarted on her rivaroxaban following the procedure.
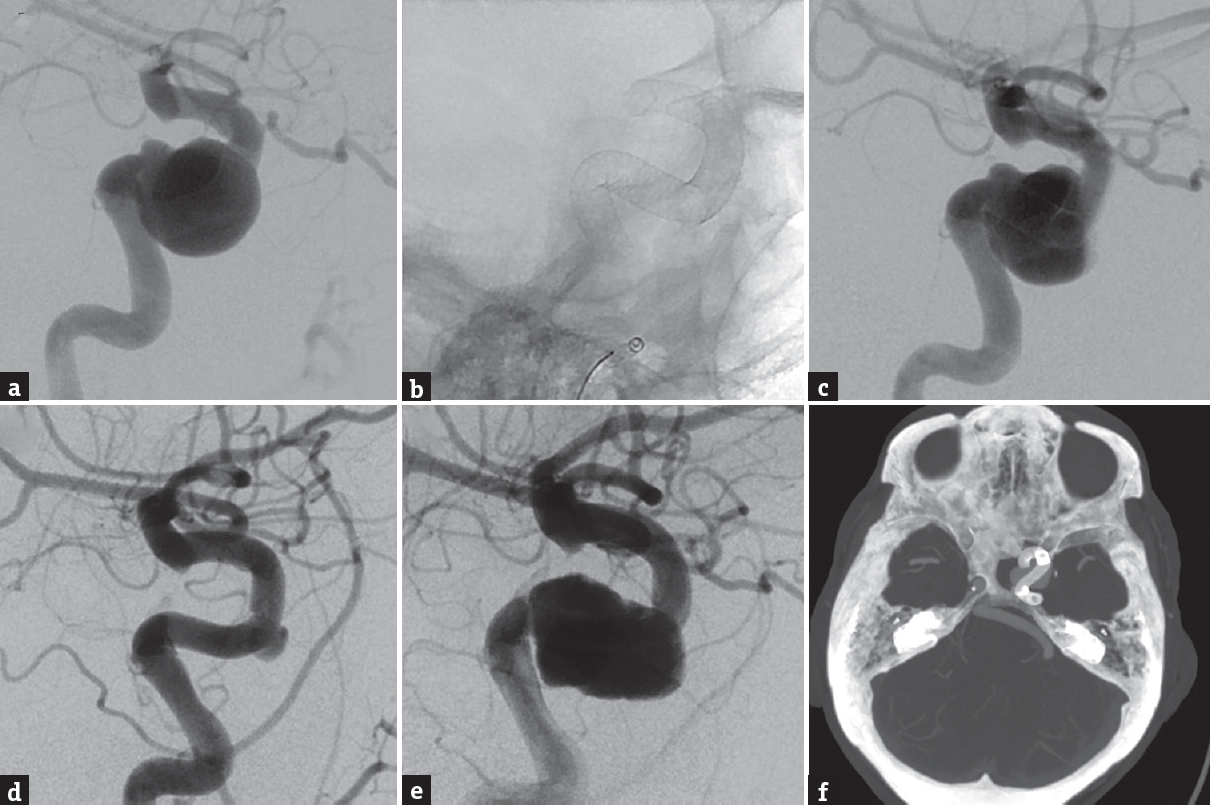
- (a) Lateral angiogram demonstrating a large 20 mm × 24 mm × 22 mm (width × depth × height) left cavernous carotid wide-necked aneurysm. (b) Lateral skull X-ray demonstrating two overlapping pipeline embolization device (4.5 mm × 20 mm and 5 mm × 20 mm, Medtronic Inc., Minneapolis, MN, USA) spanning from the left supraclinoid internal carotid artery to the vertical segment of the petrous internal carotid artery. (c) Lateral angiogram demonstrating contrast stasis within the aneurysm sac following placement of the two overlapping pipeline embolization device. (d) Lateral angiogram demonstrating significant decrease aneurysm sac filling with a small residual component measuring 7 mm × 8 mm at the anterior genu of the cavernous segment of the internal carotid artery at 4 months after pipeline embolization device placement. (e) Lateral angiogram demonstrating a large regrowth of the aneurysm, measuring 25 mm × 28 mm × 18 mm, at 10 months after pipeline embolization device placement. (f) Axial computed tomography angiogram demonstrating complete coverage of the aneurysm neck by the pipeline embolization device at 10 months after treatment
The patient returned for a follow-up angiogram at 4 months following treatment, which demonstrated remodeling of the ICA with only minimal filling of the aneurysm measuring 7 mm × 8 mm × 7 mm at the anterior genu of the cavernous segment of the ICA [Figure 1d]. Despite counseling, the patient continued to smoke during the follow-up period. In a subsequent angiogram, at 10 months after treatment, the aneurysm had regrown with interval expansion now measuring 25 mm × 28 mm × 18 mm [Figure 1e]. Computed tomography (CT) angiogram demonstrated complete PED coverage of the aneurysm neck [Figure 1f]. This patient underwent placement of another PED (5 mm × 25 mm), and interval follow-up at 3 months demonstrated persistent filling of the aneurysm.
DISCUSSION
Flow diversion is a well-established treatment for intracranial aneurysms with higher occlusion rates and similar complication rates compared to traditional embolization techniques.[2] Occlusion rates of >85%–90% at 6–12 months after PED placement have been reported in major series in the literature with low retreatment rates.[1345] In a recent study, comparing retreatment rates between PED alone versus PED and coil embolization, Park et al. reported a retreatment rate of 12% after a mean follow-up length of 9.6 months in the PED alone cohort comprising two patients with inadequate aneurysm neck coverage and six patients with persistent aneurysm filling.[3] In an earlier study comprising 101 intracranial aneurysms or dissections using the PED, Fischer reported a retreatment rate of 9% for persistent or unchanged aneurysm filling on follow-up angiography.[5] Other studies have reported similar rates of retreatment for incomplete aneurysm occlusion.[46] Despite a recent report of recurrence of a large middle cerebral artery aneurysm after a 6-month angiogram demonstrating complete occlusion after PED placement, no study in the literature has reported growth of an aneurysm after on-label PED use.[7]
Device migrations/retraction, which may result in inadequate aneurysm neck coverage by the PED, is a well-described phenomenon.[38] However, device migration/retraction was not observed in our patient, as the CT angiogram demonstrated unchanged location and configuration of the overlapping PED construct at 10-month postprocedure. Rivaroxaban use and smoking may contribute to the aneurysm regrowth and recanalization after on-label PED use. Smoking is a known risk factor of aneurysm development and subarachnoid hemorrhage (SAH), with positive correlation between the quantity of cigarettes smoked and SAH risk. In addition, smoking may be associated with aneurysm regrowth following endovascular coil embolization.[9] Similar to its effects on coiled aneurysms, smoking may preclude vessel remodeling and aneurysm sac clotting in the setting of flow diversion through its effects on vascular flow and collagen synthesis. However, data from 694 aneurysms treated using the PED have also suggested that current and former smokers have similar odds of incomplete aneurysm occlusion compared to never smokers at a mean follow-up period of 29 months.[10] Given the rarity of aneurysm regrowth after flow diversion, its risk factors may be difficult to identify.
This case represents the first reported case of aneurysm regrowth with on-label PED use after initial follow-up angiography demonstrating remodeling of the ICA and near-complete occlusion at 4 months. Despite the high aneurysm occlusion rates reported with the PED, persistent aneurysm filling and aneurysm regrowth, although rare, should not be overlooked. Although no aneurysm recurrence following complete occlusion with the on-label PED placement has been reported thus far, long-term imaging follow-up for those aneurysms may be warranted. The contribution of the rivaroxaban to regrowth in this patient should be noted, but caution should be used when counseling patients about the possibility of regrowth with this technology.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Pipeline for uncoilable or failed aneurysms: Results from a multicenter clinical trial. Radiology. 2013;267:858-68.
- [Google Scholar]
- Comparison of flow diversion and coiling in large unruptured intracranial saccular aneurysms. Stroke. 2013;44:2150-4.
- [Google Scholar]
- Re-treatment rates after treatment with the pipeline embolization device alone versus pipeline and coil embolization of cerebral aneurysms: A single-center experience. J Neurosurg. 2016;125:137-44.
- [Google Scholar]
- A single pipeline embolization device is sufficient for treatment of intracranial aneurysms. AJNR Am J Neuroradiol. 2014;35:1562-6.
- [Google Scholar]
- Pipeline embolization device (PED) for neurovascular reconstruction: Initial experience in the treatment of 101 intracranial aneurysms and dissections. Neuroradiology. 2012;54:369-82.
- [Google Scholar]
- Pipeline embolization device in treatment of 50 unruptured large and giant aneurysms. World Neurosurg. 2017;105:232-7.
- [Google Scholar]
- Recurrence of a totally occluded aneurysm after treatment with a pipeline embolization device. J Neurointerv Surg 2018 pii: Neurintsurg-2018-013842
- [Google Scholar]
- Spontaneous delayed migration/shortening of the pipeline embolization device: Report of 5 cases. AJNR Am J Neuroradiol. 2013;34:2326-30.
- [Google Scholar]
- Influence of smoking on aneurysm recurrence after endovascular treatment of cerebrovascular aneurysms. J Neurosurg. 2018;128:992-8.
- [Google Scholar]
- Smoking does not affect occlusion rates and morbidity-mortality after pipeline embolization for intracranial aneurysms. AJNR Am J Neuroradiol. 2016;37:1122-6.
- [Google Scholar]






