Translate this page into:
Astrocytic Tumors in Mexico: An Overview of Characteristics and Prognosis in an Open Reference Center for Low-Income Population
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
The authors aimed to analyze the current epidemiology of high- and low-grade gliomas, follow-up strategies, and prognosis in a national reference center of a developing country.
Materials and Methods:
Medical records of patients diagnosed with intracranial gliomas from January 2012 to January 2016 were reviewed. Data were classified by age, symptoms, Karnofsky functional scale (KFS), tumor location, extent of resection (EOR), histopathology, hospital stay, Glasgow outcome scale (GOS), adjuvant treatments, overall survival (OS), and mortality.
Results:
Astrocytomas accounted for 28.2% of the intracranial tumors and 53.5% were male. Headache was the most common symptom, while sensory disturbance was the least frequent. The right cerebral hemisphere was involved in 56.5% of cases and frontal lobe in 31.3%. Gross total resection (GTR) was achieved in 18.1% cases, 35.3% subtotal resection, and 46.4% biopsy. Regarding the astrocytomas, 43.3% were low grade and 56.4% high grade. Low-grade tumors had the highest frequency in the fourth decade of life, while Grade III and IV in the fifth and seventh decades of life, respectively. In high-grade lesions, there was a slight male predominance (~1.4:1). The initial KFS was regularly 80 for low-grade gliomas and 60 for high-grade. By 1-month postdischarge, the score decreased by 10 points. About half of the patients (47.5%) received adjuvant therapy after surgery. From the Glasgow Outcome Scale (GOS), the majority had a form of disability and 30-month OS was above 88% for Grade I-II and 0% for Grade III and IV.
Conclusions:
Astrocytic tumors were the most frequently noted intra-axial tumors. Age, histological grade, and EOR are important prognostic factors. These results are similar to other reports; however, increased variability was noted when treatment-related factors were considered. Additional studies are necessary to identify the factors related to these treatment results.
Highlights:
There are no data describing the basic epidemiology and prognosis of high-grade and low-grade gliomas in Mexico. Intracranial astrocytomas account for 28.2% tumors in our institution. Age, histological grade, and EOR are important prognostic factors. Poor overall survival was achieved in our target population.
Keywords
Brain tumors
developing countries
epidemiology
gliomas
INTRODUCTION
Currently, glioma tumors are among the most common primary intracranial tumors particularly glioblastoma, which represents the most frequent and lethal primary tumor of the central nervous system (CNS).[12] In developed countries, glioblastoma has an annual incidence of 3.2 cases per 1,000,000 inhabitants representing 12%–15% of all tumors of the CNS and 75% of malignant neurological tumors.[3] Despite their prevalence and high mortality, in Mexico, there is limited information in the literature about the epidemiology of glioblastomas.[456] Overall, there are inadequate data describing the basic epidemiology, follow-up, and prognosis of (high-grade and low-grade) gliomas in developing countries. Our main objective is to describe the current epidemiology, follow-up, and prognosis of astrocytoma patients treated in a reference center of a developing country.
MATERIALS AND METHODS
The authors reviewed the medical records of consecutive brain tumor patients from our center between January 2012 and January 2016 from the “Hospital General de Mexico” performing an observational and descriptive study. The inclusion criteria included age range of 5–80 years, newly-diagnosed cranial astrocytoma tumors confirmed by our neuropathology department, and preoperative/postoperative imaging demonstrated by computer tomography or magnetic resonance imaging (MRI).
The collated data were recorded and analyzed by age, sex, symptoms, Karnofsky functional scale (KFS), tumor location, extent of resection (EOR), tumor histopathology, hospital duration, Glasgow outcome scale (GOS), adjuvant treatments and consequent side-effects, survival, and mortality. The follow-up was for 30 months.
EOR was calculated comparing the preoperative (PO) tumor volume with the postoperative equivalent in a contrasted image study with using Osiri X v. 5.5.2 32-bit software. The results were expressed in percentages [
Supplement Figure 1
Supplement Figure 1 Three representative preoperative and postoperative tumor volumes which was analyzed using Osirix X softwareTotal resection was considered when >90% of resection was achieved, subtotal resection (STR) for 80%–90%, partial resection for 20%–80%, and biopsy when <20% of the tumor was removed.[7] The histological tumor type was described in line with the 4th edition of the World Health Organization (WHO) Classification of Tumors of the CNS[1] because WHO 2016 classification was not available at the time. The number of patients was expressed in percentages. The numerical values repeated were expressed as the mean ± standard deviation. Overall survival (OS) was plotted against tumor histology. Student's t-test was used for two-group comparison and Chi-square test for frequency distribution differences. P < 0.05 was considered statistically significant.
The case number 1 from the supplement data is presented as an illustrative case; this patient was a 35-year-old male who started 1 month ago from the diagnosis with anterograde memory impairment, disorientation, cephalalgia, and a tonic–clonic seizure, he was studied according to the neuro-oncology protocol and underwent left frontal craniotomy and resection of the lesion which was reported as a Grade III anaplastic glioma. The PO volume was 179.8 cm3 and the residual was 7.18 cm3 (3.99% of the tumor); he had a good recovery during his PO hospital staying and was discharged with for follow-up instructions, but he did not show up.
RESULTS
Patients’ lesions: Clinic and localization
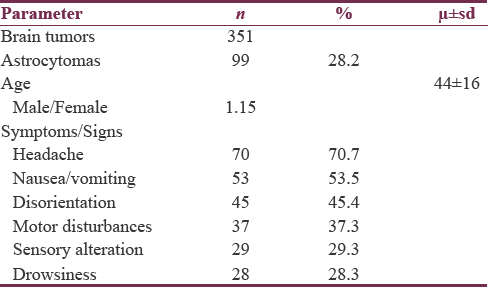
Our institution is a national reference center covering the South and South-west of Mexico, with a population of predominantly mestizo ancestry. We treated 351 patients with brain tumors within a 4-year period (2012–2016). From these patients, 99 (28.2%) were diagnosed with an astrocytic tumor. The mean age at diagnosis was 44 ± 16 years. Fifty-three (53.5%) were male and 46 (46.5%) female.
On admission, the average Karnofsky functional score (KFS) was 70–80 points. The patients with low-grade glioma (according to the WHO 2007 criteria) had higher KFS (80 points) than those with Grade III or IV astrocytomas (70 points). Regarding their clinical features, headache was the most frequent symptom in 70.7%, disorientation in 45.4%, motor disturbances in 37.3%, somnolence in 28.3%, and sensorial disturbances in 29.3% [Table 1].
From a review of the radiological images, the lesions had a predilection for the right cerebral hemisphere in 56.5% of cases. Lesions were noted to occur in the left hemisphere in 31.3%. Bihemispheric and midline distributions occurred in 9.1% and 3.0%, respectively. The most commonly affected zone was the frontal lobe in 31.3% (n = 31) of the total lesions, followed by the parietal lobe (14.1%, n = 14), cerebellum (10.1%, n = 10), and thalamus (8.1%, n = 8). In 24 cases (24.2%), tumor invasion was noted in more than one lobe, and the most common site(s) of tumor invasion were the frontoparietal lobe (12.1%, n = 12) and frontotemporal lobe (4.0%, n = 4). The areas least affected by tumor invasion were the diencephalon, midbrain, and pons with 2.0% (n = 2) each [Table 2].

Resection and histopathology
All patients underwent surgery in the Department of Neurosurgery of our hospital; and the resected lesions were analyzed by the local Department of Neuropathology. In almost one-fifth of the patients (n = 18, 18.1%), total resection was achieved and about a third (n = 35, 35.3%) underwent STR. In 46.4% (n = 46) of the cases, partial resection or biopsy was performed. According to the WHO 2007, the histopathology grades were Grade I in 16.1% (n = 16), Grade II in 27.2% (n = 27), Grade III in 17.1% (n = 17), and Grade IV in 39.3% (n = 39) [Table 2]. In the low-grade astrocytoma group, the most frequent types were pilocytic astrocytoma and diffuse astrocytoma with 10 cases (10.1%) each. In the high-grade astrocytoma group, anaplastic astrocytoma was found in 17.1% (n = 17), giant cell glioblastoma in 2.0% (n = 2), gliosarcoma in 2.0% (n = 2), and glioblastoma was diagnosed in 36.3% (n = 36) of the cases.
Postsurgery, we compared the histopathology grades with the EOR. We noted that complete resection was achieved in 27.9% of cases (n = 12) and STR was achieved in 41.9% of the cases (n = 18). However, the rates were lower in anaplastic astrocytomas (gross total resection [GTR]: 11.8%, n = 2; STR: 35.3%, n = 6) and in glioblastomas (GTR: 10.3%, n = 4; STR: 28.2%, n = 11) [Figure 1].

- Relation between histopathology grade and extension of resection in low-grade lesions GTR was 27.9% (n = 12) subtotal resection: 41.9% (n = 18); whereas in anaplastic astrocytomas GTR: 11.8%, (n = 2) subtotal resection: 35.3% (n = 6) and glioblastomas GTR: 10.3% (n = 4) subtotal resection: 28.2% (n = 11)
Histopathology by age and sex
The age incidence of low-grade astrocytomas peaked in the fourth decade of life, while the highest incidences for anaplastic astrocytomas and glioblastomas were noted in the fifth and seventh decade of life, respectively [Figure 2].

- Low-grade astrocytomas had a peak in the fourth decade of life, while anaplastic astrocytomas and glioblastomas had their highest incidence in the fifth and seventh decade of life, respectively
The average age of diagnosis was 37.5 years for low-grade lesions, 38.9 years old for anaplastic astrocytomas, and 52.4 years old for glioblastomas. On the other hand, the distribution by gender showed similar distribution between the male (53.5%) and female (46.5%) patients.
No significant difference was noted when comparing the histopathology grades in the gender groups for low-grade lesions (men: 51.1%, n = 22; women: 48.9%, n = 21). However, in cases of high-grade lesions, there was a male predilection, i.e., anaplastic astrocytoma (64.7%, n = 11 vs. 35.2%, n = 6) and glioblastoma cases (58.9%, n = 23 vs. 41.1%, n = 16). Interestingly, in the pediatric age group (5–19 years), there were three cases of high-grade lesions, i.e., two cases of anaplastic astrocytoma and one of glioblastoma.
Postoperative treatment and prognosis
After surgical resection, the mean duration of hospital stay for all astrocytoma tumors was 26.5 ± 16.2 days. However, in glioblastoma cases, the average hospital stay duration was longer (33.2 ± 19.8 days) than in those with low-grade lesions (21.5 ± 13.2 days) or those diagnosed with anaplastic astrocytomas (22.3 ± 6.8 days). At the time of discharge according to the Glasgow Outcome Scale, most of the patients were recorded with moderate disability. Low-grade astrocytomas were often in the good recovery category, while patients with high-grade lesions were mostly in the moderate and severe disability categories.
In total, half of the patients (47.5%, n = 47) received adjuvant therapy after surgery. Postoperative radiotherapy consisted of fractionated focal radiation with a total dose of 54 Gy (Gray Unit) delivered at 2 Gy/fraction for low-grade astrocytomas and 60 Gy total dose fractionated radiation at 2 Gy/fraction for high-grade lesions.
Concomitant temozolomide was added for high-grade tumors in line with the Stupp regimen. During radiotherapy, the dose administered was 75 mg/m2 of body surface area/day (7 days per week), while postradiotherapy, 6 cycles consisting of 150–200 mg/m2 of body surface area for 5 days were administered during each 28-day cycle.
In the group who received adjuvant therapy, 34 (72.3%) received radiotherapy and 13 (27.6%) were treated with chemotherapy and radiotherapy. Of the 43 patients in the low-grade astrocytoma group, 16 (37.2%) patients underwent radiotherapy and 1 (2.3%) received radiotherapy and chemotherapy.
Regarding the group of patients with anaplastic astrocytomas, 8 of 17 patients (47.0%) received radiotherapy and 1 (5.8%) received chemo-and-radiotherapy. Ten (25.6%) of the 39 glioblastoma patients received radiotherapy and 9 (23.0%) received chemotherapy and radiotherapy.
One month postdischarge, the high-grade astrocytoma group scored 50 on KFS, whereas the patients with low-grade lesions scored 70 – a lower score in comparison to the PO evaluation.
Finally, we assessed the OS according to the histopathology grade. 15-month OS for low-grade astrocytoma was recorded as 100% (Grade I) and 88% (Grade II). For anaplastic astrocytomas, the OS scores recorded were 60% and 70%. The survival rate at 30 months remained the same for low-grade astrocytomas, whereas in patients with high-grade astrocytomas, survival dropped to zero [Figure 3].

- 15-months overall survival according to the histopathology grade, for low-grade astrocytoma was 100% (grade I) and 88% (grade II); for anaplastic astrocytomas was 60% and glioblastoma 70%. The survival rate at 30 months remained in the same value for low-grade astrocytomas, whereas in high-grade astrocytomas, survival became zero
DISCUSSION
We report for the first time a general description of the epidemiology of astrocytomas (low grade and high grade) in Mexico, specifically in a region with a predominantly mestizo population.
CNS tumors are relatively uncommon tumors in adults but are the most common solid cancer in children[8] with majority associated with significant morbidity and mortality rates.[3] Astrocytoma represents the most common neoplasm in this group, and of all these astrocytomas, glioblastoma is the most common.[9]
The quality of life and vast complexity of astrocytomas have led to several institutions and groups to critically study the epidemiology data associated with these tumors[11011] and the related prognosis, reporting 5-year OS figures of 47.3% for low-grade astrocytomas[12] and 5% for high-grade gliomas, especially glioblastoma.[9]
In Mexico, brain tumors pose an important and progressively increasing dilemma. The deaths secondary to brain tumors constitute 2.5%–3% of all cancer deaths recorded from 2000 to 2011. By the end of this period, the mortality rate for brain tumors had grown by 240% in relation to the documented figures of the 1980s – this figure indicates a mortality rate of 17 deaths per million.[13] Despite this, there has been no prior description of general epidemiology in Mexico before this study.
Various authors have reported epidemiological data on astrocytic tumors in several populations with different profiles and genetic backgrounds. In adult Americans, gliomas are the second most common intracranial tumor (25%) according to the Central Brain Tumor Registry of the United States.[10] In France, it has been described with a significantly higher percentage, i.e., 48.9% making astrocytomas the most common intracranial tumor group.[11] For the Japanese population, gliomas constitute the second largest group of intracranial tumors – 19.5% of all cases.[14] The trends hint at a pattern similar to our population (where astrocytic tumors are the second most frequently diagnosed). This could be an indicator at shared pathophysiological mechanisms for the development of these neoplasms, independent of the origin of the patients. Therefore, we assume that clinical trials, novel regimens and other adjuvant therapies such as immunotherapy and tumor-treating fields could be applied to our population creating collaborations between developed and developing countries.
A few previous studies have analyzed the frequency and clinical features of brain tumors in our country[451516] demonstrating that astrocytomas always rank as one of the most common intracranial lesions, only surpassed by meningiomas. Interestingly, a recent retrospective study in a private hospital in Mexico City[16] showed that astrocytomas made up 16.8% of the CNS tumors in all age groups and was the third most common tumor. The variance in the results of the studies could be explained by the different sociogeographical characteristics of the target populations studied. In our study, the population was largely from the middle-lower social strata in the South and Southeast states, while in the study of Anaya et al. (2016), the population was chiefly from middle-upper and upper social strata of a single city. Overall, the studies exhibited several dissimilarities and although these are preliminary studies, engaging with well-structured brain tumor programs could have far-reaching effects where such discrepancies are minimized, or even totally avoided.
Several studies have shown that there is a significant correlation between EOR and the patient's survival, for example, GTRs are associated with better outcomes.[17181920] Moreover, supramaximal resection, i.e., resection beyond the zone of enhancement seen on MRI, using a subpial technique, has been associated with better OS.[202122] In our study, we observed that high-grade astrocytomas were associated with subtotal and partial resections yielding poor OS rates, while low-grade astrocytomas were associated with total resection and better survival rates, in concordance with the literature.
One of the limitations of this study is the lack of resources to grade the tumors according to the new WHO 2016, thereby hindering the utilization of possible new treatments. The same factor curbs the easy transfer of our data from our population/center to renowned centers with high per capita research outputs. We would, however, strive to analyze the gliomas diagnosed after 2016 in a future study, in line with the new classification.
Another limitation would be the reduced number of patients who completed the proposed standard treatment (radiotherapy and chemotherapy), mainly because of two factors: (1) the distance between our institution and the patient's domicile (sometimes hundreds of miles) and (2) high or unaffordable therapeutic costs for a low-economic catchment population with no health insurance in place.
CONCLUSIONS
Astrocytomas, especially glioblastomas, have become one of the most important clinical challenges in the neurosciences globally. Tackling this complex problem requires an in-depth knowledge of the profile of astrocytomas so that treatment strategies can be appropriately instituted.
We recognized that, in certain regions of the world, there remains a poor understanding of brain tumor treatments from indigent patients, inaccessible specialized centers, and suboptimal multidisciplinary programs for the best possible outcomes to be achieved.
Although it could be argued that our biomolecular analysis is not state-of-the-art, it is worthy to know that clinical epidemiological analyses like these could yet still have far-reaching positive effects in improving brain tumor-related clinical research worldwide. We believe that our work forms an educative first glance at the current management of brain tumors in a developing country, and we will be keen to repeat an improved version of this study with other centers internationally. Factors such as more research data, bigger pool of genetic diversity, and evolving patterns could provide insight not obtained anywhere else.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank Dr. Andrew F. Alalade for his support and help provided, which was greatly appreciated.
REFERENCES
- The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97-109.
- [Google Scholar]
- WHO Classification of Tumours of the Central Nervous System (4th ed). Lyon: IARC Press; 2016.
- Brain tumors in Mexico: Characteristics and prognosis of glioblastoma. Surg Neurol. 2000;53:157-62.
- [Google Scholar]
- Incidence of malignant gliomas in Mexican social service institute population from Veracruz, Mexico. Arch Neurocien. 2004;9:113-7.
- [Google Scholar]
- Survival pronostic factors in Mexican patients with multiforme glioblastoma. Rev Med Inst Mex Seguro Soc. 2010;48:121-6.
- [Google Scholar]
- Controversies in Neuro-Oncology, Best Evidence Medicine for brain Tumor Surgery. New York: Thieme; 2014. p. :1-23.
- Survival in glioblastoma: A review on the impact of treatment modalities. Clin Transl Oncol. 2016;18:1062-71.
- [Google Scholar]
- CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the united states in 2008-2012. Neuro Oncol. 2015;17(Suppl 4):iv1-62.
- [Google Scholar]
- French brain tumor database: 5-year histological results on 25 756 cases. Brain Pathol. 2011;21:633-44.
- [Google Scholar]
- Life beyond a diagnosis of glioblastoma: A systematic review of the literature. J Cancer Surviv. 2017;11:447-52.
- [Google Scholar]
- Perfil Epidemiologico de los Tumores Malignos en México. Available from: http://www.sinave.gob.mx2011
- Epidemiological study of primary intracranial tumors: A regional survey in Kumamoto prefecture in Southern Japan–20-year study. Int J Clin Oncol. 2011;16:314-21.
- [Google Scholar]
- Historical distribution of central nervous system tumors in the mexican national institute of neurology and neurosurgery. Salud Publica Mex. 2016;58:171-8.
- [Google Scholar]
- Prevalence of central nervous system tumours and histological identification in the operated patient: 20 years of experience. Cir Cir. 2016;84:447-53.
- [Google Scholar]
- Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62:753-64.
- [Google Scholar]
- Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009;110:156-62.
- [Google Scholar]
- A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190-8.
- [Google Scholar]
- The butterfly effect on glioblastoma: Is volumetric extent of resection more effective than biopsy for these tumors? J Neurooncol. 2014;120:625-34.
- [Google Scholar]
- The influence of maximum safe resection of glioblastoma on survival in 1229 patients: Can we do better than gross-total resection? J Neurosurg. 2016;124:977-88.
- [Google Scholar]
- The survival advantage of “Supratotal” resection of glioblastoma using selective cortical mapping and the subpial technique. Neurosurgery. 2017;81:275-88.
- [Google Scholar]






