Translate this page into:
Lipoprotein-Associated Phospholipase A2 is Linked with Poor Cardio-Metabolic Profile in Patients with Ischemic Stroke: A Study of Effects of Statins
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives:
The objective of the study is to investigate the effects of statins on the lipoprotein-associated phospholipase A2 (Lp-PLA2) mass in patients with ischemic stroke.
Materials and Methods:
A total number of 59 patient ages 43–69 years with cerebral stroke compared to 39 healthy controls that matching the age and body weight. The patients were divided into 32 patients on statins therapy assigned as statins users and 27 patients, not on statins therapy assigned as nonstatins users. Anthropometric and biochemical measurements were done including lipid profile and inflammatory biomarkers.
Results:
Stroke patients on statins therapy showed a comparable low of Lp-PLA (29.82 ± 3.19 IU/mL) to nonstatins user stroke patients (15.58 ± 5.73 IU/mL). Lp-PLA2 mass levels were positively correlated with body mass index, blood pressure changes, total cholesterol, triglyceride, and very low-density lipoprotein and stroke risk (SR) percentage.
Conclusions:
Patients on statins with ischemic stroke had low levels of Lp-PLA2 mass levels compared to nonstatins user with ischemic stroke. Lp-PLA2 mass levels were higher in men than women and correlated with lipid profile and SR in patients with ischemic stroke.
Keywords
Cerebral stroke
ischemic stroke
lipoprotein-associated phospholipase A2 serum levels
statins
INTRODUCTION
Stroke is the second cause of death worldwide, that accounting for about 11% of total death.[1] From 1990 to 2010, the number of strokes declined by about 10%. Most of the strokes happened in those over 65 years old. The threat of stroke augments exponentially from 30 years of age, 95% of strokes take place at age 45 years and older, however, it can occur at any age including childhood.[2] In addition, 40% of global stroke deaths are more occurred in South Asians old peoples.[3] Stroke is more common in male than female by about 25% but 60% of stroke-induced death is more in female than male.[4] Stroke is a medical emergency status due to reducing brain blood flow results in brain ischemia and cellular death. Stroke is divided into two types thrombotic stroke 85% and hemorrhagic stroke 15% while; stroke that caused by idiopathic cause is called cryptogenic stroke.[5] Stroke leads to focal brain dysfunction depending on the affected area that presented as motor and/or sensory deficits. If these deficits last <24 h is called transient ischemic attack (TIA) or mini-stroke but if lasting >24 h is called complete stroke.[6]
The most important risk factors for stroke are hypertension, obesity, dyslipidemia, smoking, and diabetes mellitus.[7]
Stroke leads to brain tissue hypoxemia which if continue >3 h lead to irreversible brain infarction. Brain ischemia activates neuronal anaerobic metabolism that provokes lactic acid production which itself damage cerebral cells causing secondary ischemic area called ischemic penumbra.[8] During ischemia, energy, and adenosine triphosphate declined to cause neuronal cell membrane damage and interruption of ion pumping. These changes activate the release of excitatory neurotransmitters such as glutamate which act on the specific receptor called N-methyl-D-aspartate receptors causing neuronal cell death.[9] Indeed, neuronal and endothelial damage activate the release of pro-inflammatory mediators which lead to the development of brain edema which further leads to the brain injury.[10] Therefore, ischemic stroke triggers a cascade of pathological episodes including neuronal excitability, intracellular calcium overload, ion homeostasis disturbances, lipid peroxidation, and free radical productions.[11] These events may occur in a time-dependent manner as neuronal excitability happens within minutes while; the inflammatory changes occur within hours after stroke. These sequential events might explain the differences in the levels of inflammatory biomarkers following stroke attacks.[12] Inflammation plays a crucial role in the pathophysiology of ischemic stroke since; neuronal damage provokes the release of inflammatory-related molecules which activate the immune system. These inflammatory mediators could escape into cerebrospinal fluid and bloodstream thus; these mediators could be simply being detectable following ischemic stroke.[13]
Lipoprotein-associated phospholipase A2 (Lp-PLA2) is a phospholipase A2 enzyme encoded by the PLA2G7 gene, it is one of different platelet-activating factors acetylhydrolase, it is 45-kDa of 441 amino acids. Lp-PLA2 is mainly combined with low-density lipoprotein (LDL) in about 80% and less with high-density lipoprotein (HDL) in about 20%. Lp-PLA2 is produced by inflammatory cells which are responsible for hydrolysis of oxidized LDL phospholipids.[14]
Furthermore, Lp-PLA2 is correlated with atherosclerosis since; it produced from the atherosclerotic plaque also; it regarded as a biomarker of cardiac diseases. In addition, Lp-PLA2 was observed as a novel biomarker for coronary artery disease and ischemic stroke.[15] High Lp-PLA2 serum levels are associated with two folds increase in the risk of stroke and poor outcomes, thus; Lp-PLA2 emerges as an excellent predictor of the incidence of stroke.[16]
Statins are 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors; inhibit de novo cholesterol biosynthesis and LDL levels as well as triglyceride (TG). Statins are effective for prevention of ischemic heart disease and cerebrovascular accidents through LDL-dependent and LDL-independent effects.[17] On the other hand, most of the statins produced differential effects on Lp-PLA2 activity and mass with a comparable reduction in LDL serum levels. On the contrary, pravastatins increases Lp-PLA2 activity and reduces Lp-PLA2 mass.[18]
Therefore, the objective of the present study was to investigate the effect of statins on the Lp-PLA2 mass in patients with ischemic stroke.
MATERIALS AND METHODS
In this cross-sectional study, a total number of 59 patient ages 43–69 years (28 males + 31 females) with cerebral stroke were recruited from stroke unite compared to 39 healthy controls that matching the age and body weight. All stroke patients were diagnosed by a consultant neurologist and they kept under supervision. Full medical history and detailed current pharmacotherapy were taken from each patient. Those patients were divided into two groups: Group A - 32 patients (17 males + 15 females) previously and currently on statins therapy assigned as statins users. Group B - 27 patients (11 males + 16 females) not on statins therapy assigned as nonstatins users. All patients were selected according to the guidelines and diagnostic criteria of the American Academy of Neurology.[19]
The study procedures were done in harmony to the Declaration of Helsinki. All patients and healthy subjects gave an informed verbal consent for their participation in this study. The study was approved by Ethical Committee and Clinical Research Editorial Board, College of Medicine, Al-Mustansiriyia University, Baghdad, Iraq from June to September 2017.
Inclusion criteria
Patients with recent cerebral strokes admitted to the stroke unite with or without statins therapy.
Exclusion criteria
Exclusion criteria were as follows hemorrhagic stroke, diabetes mellitus, thyroid disorders, valvular heart diseases, acute and chronic liver diseases, acute and chronic kidney disorders, rheumatic and connective tissues diseases, sepsis, acute and chronic infections, complicated stroke, psychiatric, and mental disorders.
Anthropometric measurements
Body weight, height, and body mass index (BMI) were recorded, BMI = Weight (kg)/Height (m2). Systolic and diastolic blood pressures were recorded at supine position from the left arm by automated digital sphygmomanometer.
Pulse pressure = SBP-DBP, mean arterial pressure =  .[20]
.[20]
Biochemical measurements
After an overnight fasting, 10 mL of venous blood sample were drown by sterile needle and plastic syringe from antecubital area from each patient at morning, 5 mL put into plain tube for routine investigations and 5 mL put into ethylenediaminetetraacetic acid tube for the assessment of the inflammatory biomarkers.
Measurement of lipid profile and fasting blood glucose
Lipid profile including total cholesterol (TC), TG, and HDL were measured by specific ELISA kits methods. Very LDL (VLDL) = TG/5, LDL was calculated according to the Friedewald formula.[21] Atherogenic index (AI) = log (TG/HDL), atherogenic coefficient (AC) = TC-HDL/HDL and cardiac risk ratio (CRR) = TC/HDL.[22]
Fasting glucose in mg/dL was measured by glucometer while HbA1c was measured by glycated hemoglobin A1C (ELISA Kit, MB845, BIOVISION, China).
Measurement of inflammatory markers
The blood samples were centrifuged for 10 min and the separated sera were used for evaluation of Lp-PLA2 serum concentration which was the mass level of Lp-PLA2 by specific ELISA kit method in IU/mL (SEA867Hu, Double-antibody Sandwich, Wuhan USCN Business Co. Ltd, China). While high sensitive C-reactive protein (CRP) serum levels were assessed by specific ELISA kit method in mg/dL (Cat. No. ABIN1115432, Wuhan USCN Business Co. Ltd, China). Each sample was calculated twice and mean of two values was estimated to reduce the errors.
Statistical analysis
Data analysis was prepared using Statistical Package for the Social Sciences (IBM, Statics for Window, Version 20.00; Armonk, NY, USA: IBM Corp). Results are presented as mean ± SD, percentage, and number. Unpaired Student's t-test was used for determination of the significant difference between two different groups, while two-way ANOVA test was used for assessing the differences among different groups. Pearson correlation was used for estimating the correlation of Lp-PLA2 serum concentration with other study parameters. The differences were significant when P < 0.05.
RESULTS
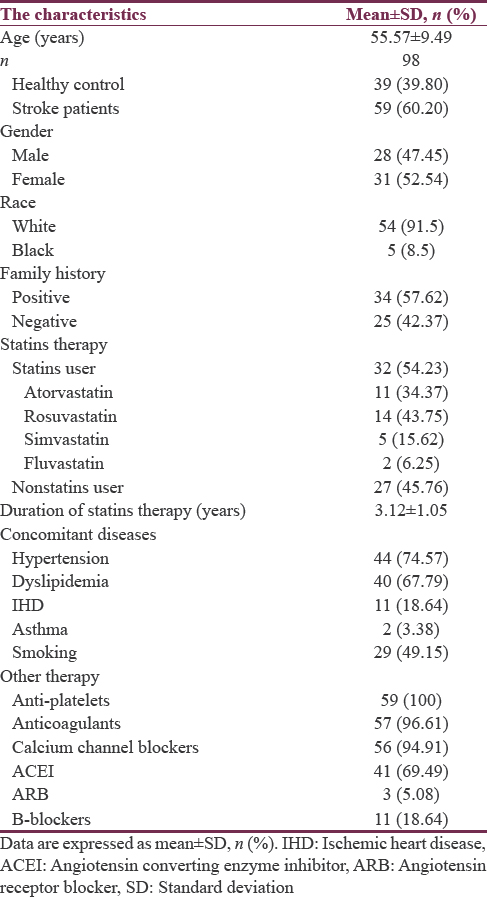
A total number of 98 recruited subjects, 60.20% of them were patients with stroke and 39.80% were healthy regarded as control. Regarding the gender and race in the present study 52.54% and 47.45% were female and male respectively with high percentage of white race 91.5% compared to the black race 8.5%. The patients with stroke had positive family history in about 57.62% compared to 42.37% with negative family history. In the present study, the stroke patients currently on statins therapy were 54.23% compared to 45.76% of the stroke patients not on statins therapy. The duration of statins therapy was 3.12 ± 1.05 years. In addition, many associated diseases were recorded in those stroke patients, including hypertension 74.57%, dyslipidemia 67.79%, ischemic heart diseases 18.64%, and asthma 3.38%. Other pharmacotherapy and characteristics have been illustrated in Table 1.
With regard to the cardio-metabolic profile, statins users showed better cardio-metabolic profile than nonstatins users in AI, AC, CRR, and similar High-sensitivity CRP (hsCRP) (P > 0.05) to the healthy controls. However, other parameters were significantly differed P < 0.01. Statins therapy illustrated an improvement in lipid profile, AI, AC, CRR and stroke risk (SR) compared to nonstatins user P < 0.01. Insignificant differences between statins and nonstatin user were observed in BMI and blood pressure levels. Moreover, stroke patients on statins therapy showed comparable low of Lp-PLA (29.82 ± 3.19 IU/mL) to nonstatins user stroke patients (15.58 ± 5.73 IU/mL) [Table 2].

Lp-PLA2 mass levels were positively correlated with BMI, blood pressure changes, TC, TG, VLDL, AC, CCR, SR %, and HsCRP, but it negatively correlated with HDL serum levels in statins user stroke patients. On the other hand, nonstatins user showed high hsCRP which illustrated high correlation with Lp-PLA2 mass P < 0.01 compared to P < 0.05 in statins user [Table 3].

In the present study, univariate logistic regression illustrated that Lp-PLA2 is an independent predictor for SR in patients with poor metabolic profile [Table 4].

Furthermore, the differential effects of statins on Lp-PLA2 mass levels were 9.8 ± 2.5 in atorvastatin, 8.95 ± 2.6 in rosuvastatin, 10.11 ± 2.1 in simvastatin, and 12.9 ± 3.1 in fluvastatin-treated patients; thus, rosuvastatin produced the powerful reduction effect on Lp-PLA2 mass levels compared to other types of statins but not to the statistically significant level P > 0.05 [Figure 1].

- Differential effect of statins on lipoprotein-associated phospholipase A2 mass levels
Besides, there was gender difference on Lp-PLA2 mass levels in patients with ischemic stroke. Male patient had a higher Lp-PLA2 mass levels than female patients in both statins and nonstatins users but the differences were nonsignificant [P > 0.05, Figure 2].

- Gender differences in lipoprotein-associated phospholipase A2 mass levels in patients with ischemic stroke
DISCUSSION
Stroke remains the main cause of permanent disability and death worldwide.[23] Inflammation plays an important role in the development of ischemic stroke by relative unknown mechanism since; stroke may possibly break the equilibrium between the inflammatory and pro-inflammatory reactions.[24] Therefore, inhibition of inflammatory reactions could lessen and recover the neurological functions. Moreover, inhibition of systemic inflammation might affect the subsequent outcomes and could deteriorate and worsen cerebral repair and functional recovery following ischemic stroke. LDL-associated Lp-PLA2 is linked with the atherogenic potential while; HDL-associated Lp-PLA2 although at low levels, play an important role in anti-atherogenic effects.[25]
In the present study, patients with ischemic stroke were linked with a poor cardio-metabolic profile as presented with dyslipidemia, hypertension, and overweight as inconsistent with Strazzullo et al., a study that illustrated obesity and overweight are linked with increased risk of ischemic stroke.[26] In contrast, Doehner et al. reported a protective role of obesity and overweight on stroke outcome including functional recovery and reducing mortality rate.[27] Therefore, obesity paradox may protect against metabolic complications in stroke patients.[28] In the current study, most of the stroke patients were hypertensive with poor therapeutic control as in agreement with animal model study of Domin et al., a study that illustrated the hypertension as an important risk factor for ischemic stroke.[29]
Moreover, Sultan et al. showed the strong association between dyslipidemia and SR as in the present study.[30]
Many observational studies illustrated the associations between lipid biomarkers mainly TC, LDL, and low HDL with the incidence of ischemic stroke due to atherogenic effect, prothrombotic changes, and abnormalities in fibrinolytic cascade.[3132]
Our results revealed elevated high-sensitive CRP levels in patients with ischemic stroke compared to healthy controls since; an increment in hsCRP serum levels are associated with high risk of cerebrovascular events due to inhibition of endothelial nitric oxide. Stroke patients on statins therapy revealed comparable low hsCRP compared to the nonstastins users which might be due to the anti-inflammatory effect of statins. In addition, hsCRP serum levels are regarded as a predictor factor for the incidence and recurrence of ischemic stroke.[33] On the other hand, Guo et al., study exposed that high CRP at admission predicts poststroke complications.[34]
Interestingly, Lp-PLA2 mass serum levels in the present study were high in stroke patients compared to the controls as demonstrated by recent Tian et al., a study that showed the association between high, Lp-PLA2 mass serum levels but not Lp-PLA2 activity with the SR.[35] This is the main reason for selection of Lp-PLA2 mass serum levels but not Lp-PLA2 activity in the present study to evaluate the potential inflammatory role in the ischemic stroke as Lp-PLA2 mass serum levels showed less time-dependent biological variability compared to Lp-PLA2 activity. Moreover, levels of Lp-PLA2 activity are linked with TIA and complicated vascular events whereas; Lp-PLA2 mass levels are more specific and accurate than Lp-PLA2 activity in the association with SR in general populations.[36] The findings of the current study pointed out that patient with ischemic stroke showed elevated levels of Lp-PLA2 mass compared to the controls as corresponding with Polupanov et al., a study that demonstrated a significant association between high Lp-PLA2 mass levels and ischemic stroke.[37] Likewise, there is a higher expression of Lp-PLA2 and its products in carotid plaque in patients with ischemic stroke. Therefore, higher Lp-PLA2 mass levels were linked with the development and progression of atherosclerotic changes which are responsible for atherogenesis and ischemic stroke.[38] Many studies revealed significant differences in Lp-PLA2 mass levels in relation to the ethnicity[39] but in this study, ethnic differences were minimally affected results of the present study since; 91.5% of our patients were white race compared to 8.5% of the black race.
Definitely, the present study suggested that high Lp-PLA2 mass levels could possibly be used as a biomarker for ischemic stroke since; it regarded as SR stratification mainly in Chines and general populations.[40] Findings of our study certainly revealed that statins therapy produced significant reduction in Lp-PLA2 mass levels in statins user patients compared to the nonstatin users in patients with ischemic stroke that correspond with Safarova et al., study that revealed a significant effect of statins on the reduction of Lp-PLA2 mass levels in patients with progressive atherosclerosis.[41] On contrast, Otsuka et al. showed insignificant effects of statins on Lp-PLA2 activity at atherosclerotic lesions which necessities the addition of anti-inflammatory agents for further diminution of Lp-PLA2 activity for prevention the progression of atherosclerotic lesions.[42] Moreover, evidence indicates that Lp-PLA2 is as well present in central nervous system microglia thus; central inhibition of Lp-PLA2 activity by statins or Lp-PLA2 selective inhibitor (GSK2647544) may lead to significant amelioration of cognitive function following ischemic stroke.[43]
Furthermore, different statins including simvastatin, fluvastatin, lovastatin, and atorvastatin lessen Lp-PLA2 mass serum levels in correspond with the reduction of LDL-cholesterol serum levels[44] but pravastatin might increase the serum levels of Lp-PLA2 mass by unknown mechanism.[45] IN addition, Ren et al.'s study demonstrated the potential effect rosuvastatin in reduction of Lp-PLA2 mass serum levels due to reduction of total LDL or to the preferential reduction effect of Lp-PLA2 mass serum levels.[46] As well, simvastatin reduce LDL-associated Lp-PLA2 through reduction of LDL and augmentation the clearance of LDL-associated enzyme.[47] These findings are in consistent with findings of the present study that illustrated the potent effect of rosuvastatin compared to the lowest effect of simvastatin which might be due to the half-life of different statins.[48] Moreover, rosuvastatin improves endothelial function, and inflammatory biomarkers and vaspin serum levels in obese patients[49] which might explain it ameliorative effect on Lp-PLA2 mass serum levels in patients with ischemic stroke.
Concerning the gender differences in Lp-PLA2 mass serum levels, the male showed a higher level compared to the female in both statin and nonstatins users. Similar results were revealed by different studies[5051] in contrast a recent Lu et al., study by disclosed high inflammatory biomarkers in women compared to male following cardiovascular ischemic disorders.[52]
Therefore, the present study gave an important innovative insight that ischemic stroke patients had higher Lp-PLA2 levels compared to the healthy controls which also correlated with poor cardio-metabolic disorders and SR. Statins significantly reduce Lp-PLA2 levels and reduce SR.
CONCLUSIONS
Patients on statins with ischemic stroke had low levels of Lp-PLA2 mass levels compared to a nonstatins user with ischemic stroke. Lp-PLA2 mass levels were high in men than women and correlated with lipid profile and SR in patients with ischemic stroke.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors kindly acknowledge Professor Sadiq M. Al-Hamash Head of, Al-Mustansiriyia University for his great support in this study.
REFERENCES
- Potential serum biomarkers in the pathophysiological processes of stroke. Expert Rev Neurother. 2014;14:173-85.
- [Google Scholar]
- Health-related quality of life and its determinants in paediatric arterial ischaemic stroke survivors. Arch Dis Child 2018 pii: archdischild-2017-313334
- [Google Scholar]
- Ethnic South Asian ischaemic stroke patients have a higher prevalence of a family history of vascular disease compared to age, gender and diabetes-matched ethnic Chinese subjects. J Neurol Sci. 2009;285:118-20.
- [Google Scholar]
- Sex as predictor for achieved health outcomes and received care in ischemic stroke and intracerebral hemorrhage: A register-based study. Biol Sex Differ. 2018;9:11.
- [Google Scholar]
- Patent foramen ovale closure or medical therapy for cryptogenic ischemic stroke: An updated meta-analysis of randomized controlled trials. Clin Res Cardiol 2018:2. Epub ahead of print
- [Google Scholar]
- The clinical features of ischemic stroke patients for whom smoking was considered the sole risk factor for ischemic stroke. Intern Med. 2018;15(57):1703-6.
- [Google Scholar]
- Biological age is a predictor of mortality in ischemic stroke. Sci Rep. 2018;8:4148.
- [Google Scholar]
- Lactate and the lactate-to-pyruvate molar ratio cannot be used as independent biomarkers for monitoring brain energetic metabolism: A microdialysis study in patients with traumatic brain injuries. PLoS One. 2014;9:e102540.
- [Google Scholar]
- High glucose induces apoptosis and suppresses proliferation of adult rat neural stem cells following in vitro ischemia. BMC Neurosci. 2013;14:24.
- [Google Scholar]
- The role of antiplatelet therapy in carotid stenting for ischemic stroke prevention. Stroke. 2006;37:1572-7.
- [Google Scholar]
- Redox status in acute ischemic stroke: Correlation with clinical outcome. Mol Cell Biochem. 2015;406:75-81.
- [Google Scholar]
- Augmentation of M-type (KCNQ) potassium channels as a novel strategy to reduce stroke-induced brain injury. J Neurosci. 2015;35:2101-11.
- [Google Scholar]
- Fullerenols and glucosamine fullerenes reduce infarct volume and cerebral inflammation after ischemic stroke in normotensive and hypertensive rats. Exp Neurol. 2015;265:142-51.
- [Google Scholar]
- Association of lipoprotein-associated phospholipase A2 mass with asymptomatic cerebral artery stenosis. J Cell Mol Med. 2018;22:2329-36.
- [Google Scholar]
- Lipoprotein-associated phospholipase A2 and risk of incident peripheral arterial disease: Findings from the atherosclerosis risk in communities study (ARIC) Atherosclerosis. 2018;268:12-8.
- [Google Scholar]
- Prognostic value of lipoprotein-associated phospholipase A2 mass for all-cause mortality and vascular events within one year after acute ischemic stroke: Methodological issues. Atherosclerosis. 2018;268:231-2.
- [Google Scholar]
- Acylation-stimulating protein is a surrogate biomarker for acute myocardial infarction: Role of statins. J Lab Physicians. 2017;9:163-9.
- [Google Scholar]
- Lipoprotein-associated phospholipase A2 activity and low-density lipoprotein subfractions after a 2-year treatment with atorvastatin in adolescents with type 1 diabetes. J Pediatr Endocrinol Metab. 2016;29:1181-6.
- [Google Scholar]
- American College of Emergency Physicians, American Academy of Neurology. Clinical policy: Use of intravenous tPA for the management of acute ischemic stroke in the emergency department. Ann Emerg Med. 2013;61:225-43.
- [Google Scholar]
- Pulse pressure and mean arterial pressure in relation to ischemic stroke among patients with uncontrolled hypertension in rural areas of China. Stroke. 2008;39:1932-7.
- [Google Scholar]
- Validation of the Friedewald formula for the estimation of low density lipoprotein cholesterol in a sub-Saharan African population. Clin Biochem. 2018;53:25-30.
- [Google Scholar]
- Effects of rosuvastatin alone or in combination with omega-3 fatty acid on adiponectin levels and cardiometabolic profile. J Basic Clin Pharm. 2016;8:8-14.
- [Google Scholar]
- Circadian rhythm of onset of stroke-in 50 cases of ischemic stroke. Mymensingh Med J. 2015;24:121-6.
- [Google Scholar]
- Cell death mechanisms in stroke and novel molecular and cellular treatment options. Curr Neuropharmacol 2018:1. Epub ahead of print
- [Google Scholar]
- Salubrinal and robenacoxib treatment after global cerebral ischemia. Exploring the interactions between ER stress and inflammation. Biochem Pharmacol. 2018;151:26-37.
- [Google Scholar]
- Excess body weight and incidence of stroke: Meta-analysis of prospective studies with 2 million participants. Stroke. 2010;41:e418-26.
- [Google Scholar]
- Overweight and obesity are associated with improved survival, functional outcome, and stroke recurrence after acute stroke or transient ischaemic attack: Observations from the TEMPiS trial. Eur Heart J. 2013;34:268-77.
- [Google Scholar]
- Obesity paradox in stroke-myth or reality? A systematic review. PLoS One. 2017;12:e0171334.
- [Google Scholar]
- Neuroprotective effect of the group III mGlu receptor agonist ACPT-I after ischemic stroke in rats with essential hypertension. Prog Neuropsychopharmacol Biol Psychiatry. 2018;84:93-101.
- [Google Scholar]
- Dyslipidemia in children with arterial ischemic stroke: Prevalence and risk factors. Pediatr Neurol. 2018;78:46-54.
- [Google Scholar]
- Lipid and lipoprotein biomarkers and the risk of ischemic stroke in postmenopausal women. Stroke. 2012;43:958-66.
- [Google Scholar]
- High sensitivity C-reactive protein levels in acute ischemic stroke and subtypes: A study from a tertiary care center. Iran J Neurol. 2013;12:92-7.
- [Google Scholar]
- Elevated CRP at admission predicts post-stroke cognitive impairment in Han Chinese patients with intracranial arterial stenosis. Neurol Res. 2018;16:1-5.
- [Google Scholar]
- The associations of stroke, transient ischemic attack, and/or stroke-related recurrent vascular events with lipoprotein-associated phospholipase A2: A systematic review and meta-analysis. Medicine (Baltimore). 2017;96:e9413.
- [Google Scholar]
- Biological variability of lipoprotein-associated phospholipase A2 activity in healthy individuals. Clin Biochem. 2017;50:347-9.
- [Google Scholar]
- Lipoprotein-associated phospholipase A2: Relation to development of ischemic stroke in patients with essential hypertension. Kardiologiia. 2014;54:29-34.
- [Google Scholar]
- Treatment with beta-blockers is associated with lower levels of lp-PLA2 and suPAR in carotid plaques. Cardiovasc Pathol. 2013;22:438-43.
- [Google Scholar]
- Association of lipoprotein-associated phospholipase A (2) and endothelial function in the multi-ethnic study of atherosclerosis (MESA) Vasc Med. 2011;16:247-52.
- [Google Scholar]
- Association of lp-PLA2 G994T gene polymorphism with risk of ischemic stroke in Chinese population. J Biochem Mol Toxicol. 2017;31:244-51.
- [Google Scholar]
- Pleiotropic effects of nicotinic acid therapy in men with coronary heart disease and elevated lipoprotein (a) levels. Kardiologiia. 2011;51:9-16.
- [Google Scholar]
- Community-based statins and advanced carotid plaque: Role of CD163 positive macrophages in lipoprotein-associated phospholipase A2 activity in atherosclerotic plaque. Atherosclerosis. 2017;267:78-89.
- [Google Scholar]
- Evaluation of the safety, pharmacokinetics, pharmacodynamics, and drug-drug interaction potential of a selective lp-PLA2 inhibitor (GSK2647544) in healthy volunteers. Int J Clin Pharmacol Ther. 2016;54:935-49.
- [Google Scholar]
- Fluvastatin slow-release lowers platelet-activating factor acetyl hydrolase activity: A placebo-controlled trial in patients with type 2 diabetes. J Clin Endocrinol Metab. 2004;89:1153-9.
- [Google Scholar]
- Lipoprotein-associated phospholipase A2 and its association with cardiovascular outcomes in patients with acute coronary syndromes in the PROVE IT-TIMI 22 (PRavastatin or atorVastatin evaluation and infection therapy-thrombolysis in myocardial infarction) trial. Circulation. 2006;113:1745-52.
- [Google Scholar]
- Comparison of the effect of rosuvastatin versus rosuvastatin/ezetimibe on markers of inflammation in patients with acute myocardial infarction. Exp Ther Med. 2017;14:4942-50.
- [Google Scholar]
- Simvastatin reduces lipoprotein-associated phospholipase A2 in lipopolysaccharide-stimulated human monocyte-derived macrophages through inhibition of the mevalonate-geranylgeranyl pyrophosphate-rhoA-p38 mitogen-activated protein kinase pathway. J Cardiovasc Pharmacol. 2011;57:213-22.
- [Google Scholar]
- Rosuvastatin improves vaspin serum levels in obese patients with acute coronary syndrome. Diseases. 2018;6:pii: E9.
- [Google Scholar]
- Lipoprotein-associated phospholipase A2 predicts future cardiovascular events in patients with coronary heart disease independently of traditional risk factors, markers of inflammation, renal function, and hemodynamic stress. Arterioscler Thromb Vasc Biol. 2006;26:1586-93.
- [Google Scholar]
- Lipoprotein-associated phospholipase A2 does not predict mortality or new ischaemic events in acute coronary syndrome patients. Eur Heart J. 2007;28:699-704.
- [Google Scholar]
- Sex differences in inflammatory markers and health status among young adults with acute myocardial infarction: Results from the VIRGO (Variation in recovery: Role of gender on outcomes of young acute myocardial infarction patients) study. Circ Cardiovasc Qual Outcomes. 2017;10:e003470.
- [Google Scholar]






