Translate this page into:
Use of Tramadol for Management of Opioid Use Disorders: Rationale and Recommendations
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Opioids are one of the most common illicit psychoactive substances being used in India. In fact, opioid use disorders are the most common disorder presenting to the substance use disorder treatment centers across the country. Effective and evidence-based interventions are available for management of opioid use disorders. However, the treatment for opioid use disorders remains difficult to access for most of those in need in India. The current article presents the literature on the use of tramadol for the management of opioid use disorders. It also makes recommendations on the use of tramadol for the management of opioid use disorders. Tramadol offers a viable alternative to the existing options for the management of opioid use disorders. It has been found effective when used for this indication. It offers certain major advantages such as easy and wide availability and low abuse liability. It offers a good option to expand the treatment services for opioid use disorders across the country.
Keywords
Drug abuse
opioid addiction
opioid use disorders
tramadol
INTRODUCTION
Illicit drug use disorders are a major public health problem that contributes significantly to the global burden of disease. These are responsible for 10.9% of years lived with disability globally.[1] Opioids are one of the most common illicit psychoactive substances being used in India. In fact, opioid use disorders are the most common disorder presenting to the substance use disorder treatment centers across the country.[23] A simple extrapolation of the findings of the 2004 National Survey puts the number of those with opioid dependence in the country at around 2.3 million.
Effective and evidence-based interventions are available for the management of opioid use disorders. In fact, of all the illicit psychoactive substances, it is the opioids for which the effective and approved medicines have existed for the longest period of time. Methadone was approved for use in the management of opioid dependence way back in 1972 and buprenorphine was approved for this purpose in the year 2002. However, the treatment for opioid use disorders remains difficult to access for most of those in need in India.
CHALLENGES OF THE CURRENT STATE OF TREATMENT OF OPIOID USE DISORDERS IN INDIA: NEED FOR ALTERNATIVES
Management of opioid use disorders can be considered under two phases-the initial short-term phase (of withdrawal management-detoxification) and the long-term phase. The aim of the initial short-term phase of treatment is to wean the patient off from opioids being abused in a safe and controlled manner. The long-term phase aims to prevent relapse. Currently, buprenorphine, methadone, and naltrexone are three most commonly used treatment options for the management of opioid use disorders in India. Besides these, a combination of clonidine, benzodiazepines and a nonsteroidal anti-inflammatory drug is also widely used during the short-term phase.
Opioid agonists (and partial agonists) have been found to be effective for the management of opioid use disorders in both the phases. The use of opioid agonists (and partial agonists) during the short-term phase helps address the opioid withdrawals effectively, thereby minimizing the discomfort and improving treatment completion.[4] Similarly, the use of opioid agonists or partial agonists during long-term phase of management has been found to be associated with improved treatment outcome.[56] Opioid antagonists are also available for use during the long-term phase and the limited literature form the country has identified it be a feasible option for at least a subgroup of individuals with opioid use disorders.[7]
Buprenorphine is being used for the management of opioid use disorder in the country since at least late 1980s. Methadone has been a late entrant, with its use starting in country in the year 2012. In addition, naltrexone is available in the country for many years. Scaling up of the opioid-based treatment has been carried out in the country through the efforts of academic institutions (like AIIMS, New Delhi), governmental organizations (like National AIDS Control Organization), government ministries and departments (like Drug De-addiction Programme, Ministry of Health and Family Welfare), and nongovernment organizations (like UNODC and other civil society organizations working in this area). Despite these efforts, the availability of the treatment for opioid use disorders in the country remains largely restricted. The regulatory status of buprenorphine and methadone and the risk of diversion are two of the biggest challenges to the easy availability of these medications to the end users.[8] Consequently, there is a need to explore the alternatives to these medicines that can help overcome these barriers and make the treatment accessible to a greater number of persons with opioid use disorders. This is especially true for the remote and rural locations in the country that are usually underserved by such treatment services.
Tramadol, an opioid agonist, offers one such alternative. However, the current Indian guidelines on the management of opioid use disorders do not make any recommendations on the use of tramadol.[9] The current article presents a narrative review of the literature on the use of tramadol for the management of opioid use disorders. It also makes recommendations on the use of tramadol for the management of opioid use disorders.
TRAMADOL: OVERVIEW
Tramadol hydrochloride (tramadol) ([1RS,2RS]-2- [(dimethylamino) methyl]-1-[3-methoxyphenyl]-cyclo hexanol HCl) is a centrally acting analgesic which was synthesized in 1962.
Tramadol pharmacodynamics
Tramadol produces its analgesic effects by both opioid and nonopioid mechanisms. Tramadol has a weak affinity for the mu receptors. The mu receptor agonistic activity of tramadol is almost 6000-fold less than that of morphine. However, its metabolite, M1 has 300 times more affinity for the mu receptor as compared to the parent compound of tramadol. The other mechanisms by which tramadol acts on the central nervous system includes blockade of reuptake of serotonin and norepinephrine.[10]
Tramadol pharmacokinetics
Tramadol is available in various pharmaceutical formulations including tablets, soluble tablets, capsules, drops, suppositories, and ampoules. In India, tablets and the capsules are the most commonly used and easily available formulations. Immediate release formulations require 4–6 times daily administration. Sustained release formulations are also available, and these provide an opportunity for less frequent dosing (i.e., twice a day).
After an oral administration of tramadol tablets, drug is absorbed rapidly (after a lag time of half hour for capsules) and almost completely. Peak plasma concentration of tramadol is achieved after 1.6–1.9 h of oral administration of capsules.[11] The bioavailability is around 70% after single dose administration, but this increases to 90%–100% after repeated administration probably because of saturation of hepatic first pass.[12] Tramadol crosses the blood–placental barrier. However, a very small amount of it is excreted in the maternal breast milk.
Tramadol is mainly metabolized by two pathways: N- and O-demethylation (phase I reactions) and conjugation (phase-II reactions). There are 11 known metabolites of tramadol (M1 to M5 and glucuronides and sulfates of M1, M4, and M5). Tramadol is mainly excreted through the kidneys and the rest through the fecal route.[11] The elimination half-life of the tramadol is 5–6 h.
Medical use
Tramadol, like other opioids, is commonly used for acute and chronic pain conditions. Multiple systematic reviews suggest a role of tramadol in relief of various pain conditions including osteoarthritis,[13] neuropathic pain,[14] chronic low backache,[15] cancer pain,[16] and postoperative pain.[17] Some of the salient observations with regard to the use of tramadol for these conditions have been summarized in Box 1. More recently, tramadol has also been used as a standalone treatment for premature ejaculation and has shown a good efficacy.[18]

TRAMADOL FOR MANAGEMENT OF OPIOID USE DISORDERS: CURRENT STATE OF EVIDENCE
Methodology
The electronic database of PubMed was searched for the relevant literature. We searched for the literature on the use of tramadol for the management of opioid use disorders. This included the use of tramadol for short-term detoxification as well as long-term maintenance. The search was carried out in March 2018 and included articles published till and including March 2018. The search was restricted to human studies published in English language. The MeSH search terms used included “Tramadol” AND “Opioid-Related Disorders;” AND “Tramadol” AND “Opiate Substitution Treatment.” All publication types were included in the search. The search revealed a total of 70 publications. Out of these 70 publications, six were found to be relevant for inclusion in the current review. The rest of the studies were excluded as these were not related to use of tramadol for the management of opioid use disorders. The reference list of these six studies was searched manually for other relevant publications. An additional eight publications were found making the total studies included in the current review to be 14 [Figure 1].
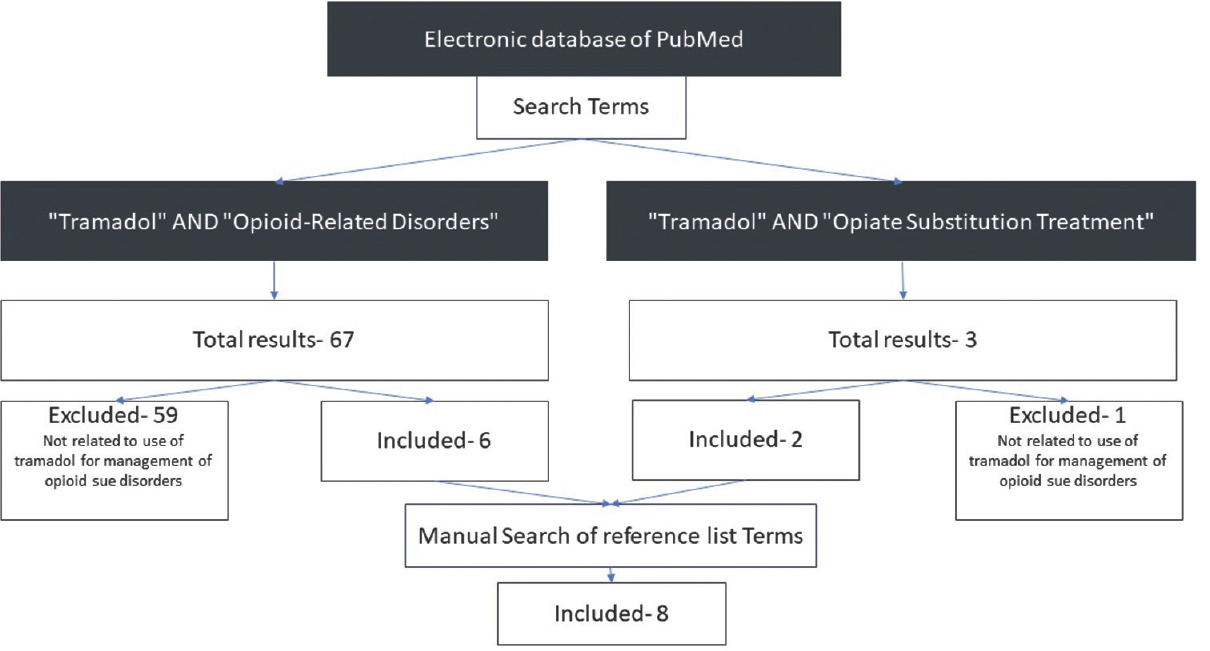
- Search strategy for the narrative review
The studies that have examined the efficacy of tramadol in the short-term phase of management of opioid use disorders (also known as detoxification). These studies have been summarized in Table 1.
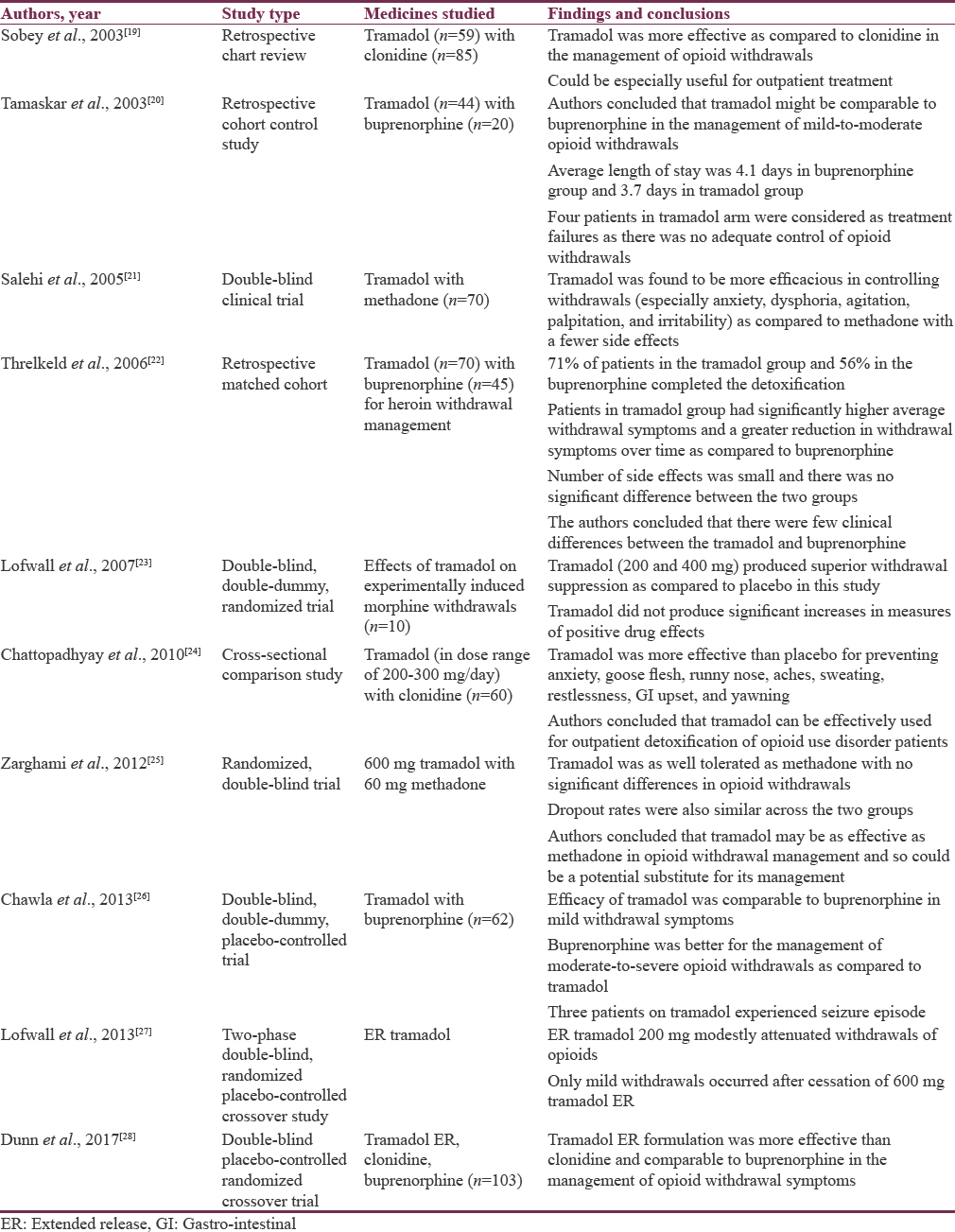
In summary, the studies suggest the superiority of tramadol over clonidine in the management of opioid withdrawals. These studies also suggest that tramadol may be equally efficacious as buprenorphine or methadone, at least for the mild-to-moderate opioid withdrawals. The side effect profile also appears to be favorable with no major differences as compared to other opioid agonists.
A limited body of literature also exists on the use of tramadol for longer periods of time in the management of opioid use disorders. Case reports exist for the use of tramadol safely for as long as 1 year among persons with opioid use disorders.[2930] Tramadol has been used extensively over the past few years for the management of opioid use disorders at our center. Our experience suggests tramadol to be a viable option for use among persons with opioid use disorders. A case series on the use of tramadol for the management of opioid use disorders reported findings from 25 patients who were prescribed tramadol for at least a period of 6 months and were on treatment for at least 80% of the time.[31] The findings suggested that patients with dependence on diverse types of opioids (natural opioids such as poppy husk, raw opium [afeem], heroin, dextropropoxyphene, and pentazocine [injection]) could be managed with tramadol. The median dose of tramadol used was 300 mg/day. While 19 patients attained complete abstinence (>1 month) from opioids being abused at least once, six attained partial abstinence (i.e., reduction of dose of opioids or abstinence for <1-month duration). Another chart review from the center has suggested that patients with greater age, longer duration of opioid use, and better follow-up adherence are more likely to be abstinent if prescribed tramadol for opioid use disorders for at least 6-month period. The rates of abstinence were higher among those presenting with natural opioid abuse as compared to prescription opioid abuse or heroin abuse.[32]
Diversion of medically prescribed tramadol
One of the reasons for limited availability of methadone and buprenorphine is the concern with the possibility of diversion. Reports on diversion of medically prescribed opioids are well documented in the literature.[33] This is due to the high street value of these medicines that is attributable of their high abuse liability. On the contrary, tramadol is considered to be an opioid with low abuse liability historically and was introduced as an unscheduled drug in many countries.
Animal studies have reported tramadol to be a weak reinforcer of self-administration compared to other pure opioid agonists like morphine.[34] Based on these observations, it has often been described as “an atypical opioid analgesic with mild opioid-like effects.” Abuse liability studies among human beings have also found it to have lesser abuse liability as compared to morphine. However, when administered to recreational drug users[35] as well as nondependent individuals,[36] tramadol is often recognized as an opioid and is reported as a “liked drug.” Interestingly, parentally administered tramadol is less likely to be identified as an opioid due to bypassing of the first-pass metabolism, thereby minimizing M1 production. Hence, abuse of tramadol through injecting route is even lesser likely. Reports on tramadol abuse have been reported in the literature. These include case reports and case series on tramadol abuse from India.[37] Epidemiological studies have reported a lower tramadol misuse as compared to other opioids.[3839] Consequently, its use is less likely to be associated with diversion. Postmarketing surveillance has indicated that tramadol has lower rates of abuse as compared to oxycodone.[40] However, data indicates a growing number of tramadol abusers in some Middle East countries.[38] Furthermore, certain formulations such as secure-release formulation of once-daily tramadol have been developed with an aim to prevent abuse inhalational route.[41]
The rationale for the use of tramadol in the management of opioid use disorders is summarized. In brief, tramadol offers an effective, easily and widely available alternative to methadone and buprenorphine for the management of opioid use disorders. Some of the biggest strengths of tramadol include easy logistics of procurement and storage due to less restrictive regulatory requirements and option of dispensing as take-home medicine with lesser risk of diversion, thereby circumventing the need for daily follow up.
RECOMMENDATIONS ON USE OF TRAMADOL FOR MANAGEMENT OF OPIOID USE DISORDERS
The recommendations on the use of naltrexone for the management of opioid use disorders have been presented in this section. Only the issues specific to the use of tramadol have been presented here. These should be supplementary to the general recommendations on the management of opioid use disorders.
Indications of use
Tramadol can be used for the management of opioid use disorders. It can be used for the management during the short-term phase (detoxification). Furthermore, tramadol can be used for prolonged detoxification (extending over a few weeks to a few months) from outpatient setting for patients who are not able to stop opioid use following short-term detoxification. Tramadol can be used for dependence on all opioids including natural opioids such as poppy husk, raw opium [afeem], heroin, dextropropoxyphene, pentazocine [injection]). However, it is likely to be more effective for lower potency opioids such as natural opioids.
Dosage of tramadol
Based on the review of the studies available on the use of tramadol in opioid use disorders and in various pain conditions, it is evident that the use of tramadol in the dose range of 300–400 mg/day is safe, with few side effects. The guidelines for the management of pain conditions suggest initiating tramadol treatment with the lower dose and increase it gradually.[4243] However, the patients of opioid use disorders, who are already tolerant to opioids, can be started with the maximum dose, that is, 300 mg to 400 mg per day from day one itself if indicated. Because of the short half-life of tramadol, it should be prescribed in 3–4 divided doses per day. If possible, shift to the extended-release or long-acting formulations of tramadol after stabilization of the daily dose.
Duration of treatment
The duration for which tramadol is prescribed depends on the treatment goal and overall management plan for the individual patient. When used for the short-term detoxification, it can be tapered off according to various regimens including 10, 14, 21, or 42 days. However, experience at our center suggests that this short-term detoxification might not work for many patients. Previous research also suggests that when the dose reductions are fast, treatment dropouts are high, and hence, the treatment effectiveness is lower in achieving long-term abstinence. Hence, in case the short-term detoxification fails or the patient/treating clinician prefers, it can be used for extended detoxification over a period of few weeks to a few months before it is gradually tapered off. Similar approach has been used previously and is referred to by many names including “methadone tapering,” “short-term maintenance,” “long-term detoxification,” “maintenance to abstinence,” “slow methadone detoxification,” and “abstinence-oriented maintenance.”[4445] In our experience, many patients prefer prolonged tramadol detoxification (over a period of 4–6 months) to the usual short-term detoxification (done over a period of 2–3 weeks) on an outpatient basis. The final pace of tapering is to be decided in discussion with the patient.
Setting of treatment
Tramadol can be used in in-patient as well outpatient setting. It can be prescribed as take-home medication if used in the outpatient setting.
Side effects to be monitored
Some of the commonly reported side effects of tramadol include nausea, dizziness, drowsiness, tiredness, fatigue, sweating, vomiting, dry mouth, and postural hypotension. One of the serious adverse effects associated with the use of tramadol is seizures. However, the seizures generally observed at higher doses (beyond the recommended maximum doses of 400 mg/day) and it rarely occurs at therapeutic doses of tramadol. Furthermore, the recurrent seizures are uncommon and the usual outcome is full recovery.[38]
In overdose, tramadol can lead to tachycardia, hypertension, seizures, coma, and respiratory depression.
Drug interactions
Concomitant use of monoamine oxidase inhibitors with tramadol is contraindicated. Caution should be exercised when used concomitantly with carbamazepine, digoxin, erythromycin, ketoconazole, lithium, mirtazapine, bupropion, fluoxetine, paroxetine, phenytoin, promethazine, rifampin, ritonavir, quinidine, trazodone, coumarins, diuretics, phenothiazines, and triptan medicines. Furthermore, one should be cautious with the concomitant use of other medicines with sedative properties.
CONCLUSIONS
Tramadol offers a viable alternative to the existing options for the management of opioid use disorders. It has been found effective when used for this indication. It offers certain major advantages such as easy and wide availability and low abuse liability. It offers a good option to expand the treatment services for opioid use disorders across the country. More evidence on its use over extended period of times will be of great utility in the future.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Global burden of disease attributable to mental and substance use disorders: Findings from the global burden of disease study 2010. Lancet. 2013;382:1575-86.
- [Google Scholar]
- A retrospective chart review of treatment seeking middle aged individuals at a tertiary care substance use disorder treatment centre in North Part of India over five successive years: Findings from drug abuse monitoring system. Scientific World Journal 2013 2013 316372
- [Google Scholar]
- The Extent, Pattern and Trends of Drug Abuse in India-National Survey. New Delhi: Ministry of Social Justice and Empowerment, Government of India and United Nations Office on Drugs and Crime; 2004.
- [Google Scholar]
- Buprenorphine for managing opioid withdrawal. Cochrane Database Syst Rev. 2017;2:CD002025.
- [Google Scholar]
- Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;3:CD002209.
- [Google Scholar]
- Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2004;3:CD002207.
- [Google Scholar]
- An open-label naturalistic study of predictors of retention and compliance to naltrexone maintenance treatment among patients with opioid dependence. J Subst Use. 2016;21:309-16.
- [Google Scholar]
- Buprenorphine and buprenorphine/naloxone diversion, misuse, and illicit use: An international review. Curr Drug Abuse Rev. 2011;4:28-41.
- [Google Scholar]
- Clinical Practice Guidelines for the Assessment and Management of Substance Use Disorders: Indian Psychiatric Society. 2014
- [Google Scholar]
- Bioavailability of enteral tramadol formulations 1st communication: Capsules. Arzneimittelforschung. 1986;36:1278-83.
- [Google Scholar]
- Tramadol for osteoarthritis: A systematic review and meta-analysis. J Rheumatol. 2007;34:543-55.
- [Google Scholar]
- Tramadol for neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6:CD003726.
- [Google Scholar]
- Opioids compared to placebo or other treatments for chronic low-back pain. Cochrane Database Syst Rev. 2013;8:CD004959.
- [Google Scholar]
- Tramadol with or without paracetamol (acetaminophen) for cancer pain. Cochrane Database Syst Rev. 2017;5:CD012508.
- [Google Scholar]
- Tramadol for postoperative pain treatment in children. Cochrane Database Syst Rev. 2015;3:CD009574.
- [Google Scholar]
- Role of tramadol in premature ejaculation: A systematic review and meta-analysis. Urol Int. 2013;91:197-205.
- [Google Scholar]
- The use of tramadol for acute heroin withdrawal: A comparison to clonidine. J Addict Dis. 2003;22:13-25.
- [Google Scholar]
- Tramadol versus buprenorphine for the treatment of opiate withdrawal: A retrospective cohort control study. J Addict Dis. 2003;22:5-12.
- [Google Scholar]
- Tramadol versus methadone for the management of acute opioid withdrawal: An add-on study. J Res Med Sci. 2005;11:185-9.
- [Google Scholar]
- Tramadol versus buprenorphine for the management of acute heroin withdrawal: A retrospective matched cohort controlled study. Am J Addict. 2006;15:186-91.
- [Google Scholar]
- Modest opioid withdrawal suppression efficacy of oral tramadol in humans. Psychopharmacology (Berl). 2007;194:381-93.
- [Google Scholar]
- Tramadol versus clonidine in management of heroin withdrawl. Asian J Psychiatr. 2010;3:237-9.
- [Google Scholar]
- Tramadol versus methadone for treatment of opiate withdrawal: A double-blind, randomized, clinical trial. J Addict Dis. 2012;31:112-7.
- [Google Scholar]
- Comparison of efficacy between buprenorphine and tramadol in the detoxification of opioid (heroin)-dependent subjects. J Opioid Manag. 2013;9:35-41.
- [Google Scholar]
- Efficacy of extended-release tramadol for treatment of prescription opioid withdrawal: A two-phase randomized controlled trial. Drug Alcohol Depend. 2013;133:188-97.
- [Google Scholar]
- Efficacy of tramadol extended-release for opioid withdrawal: A randomized clinical trial. JAMA Psychiatry. 2017;74:885-93.
- [Google Scholar]
- Tramadol for maintenance treatment for an elderly” doda”(poppy husk) user. J Geriatr Ment Health. 2016;3:179.
- [Google Scholar]
- Maintenance treatment of opioid dependence with tramadol. J Neurosci Rural Pract. 2017;8:S98-101.
- [Google Scholar]
- Tramadol for maintenance for opioid dependence: A chart review. J Opioid Manag. 2017;13:329-34.
- [Google Scholar]
- A review of buprenorphine diversion and misuse: The current evidence base and experiences from around the world. J Addict Med. 2014;8:315-26.
- [Google Scholar]
- Abuse liability, behavioral pharmacology, and physical-dependence potential of opioids in humans and laboratory animals: Lessons from tramadol. Biol Psychol. 2006;73:90-9.
- [Google Scholar]
- Profiling the subjective, psychomotor, and physiological effects of tramadol in recreational drug users. Drug Alcohol Depend. 2005;80:273-8.
- [Google Scholar]
- Discriminative stimulus effects of tramadol in humans. J Pharmacol Exp Ther. 2011;338:255-62.
- [Google Scholar]
- Tramadol dependence: A case series from India. Indian J Psychol Med. 2012;34:283-5.
- [Google Scholar]
- The diversion of ultram, ultracet, and generic tramadol HCL. J Addict Dis. 2006;25:53-8.
- [Google Scholar]
- World Health Organization. Tramadol. Update Review. Proceedings from the Expert Committee on Drug Dependence, Thirty Sixth Meeting, Geneva; 16-20 June. 2014
- [Google Scholar]
- Update on abuse-resistant and abuse-deterrent approaches to opioid formulations. Pain Med. 2009;10(Suppl 2):S124-33.
- [Google Scholar]
- Rates of abuse of tramadol remain unchanged with the introduction of new branded and generic products: Results of an abuse monitoring system, 1994-2004. Pharmacoepidemiol Drug Saf. 2005;14:851-9.
- [Google Scholar]
- CDC guideline for prescribing opioids for chronic pain-United States, 2016. JAMA. 2016;315:1624-45.
- [Google Scholar]
- Canadian guideline for safe and effective use of opioids for chronic noncancer pain: Clinical summary for family physicians. Part 1: General population. Can Fam Physician. 2011;57:1257-66. e407-18
- [Google Scholar]
- Retention in methadone maintenance and heroin addicts’ risk of death. Addiction. 1994;89:203-9.
- [Google Scholar]
- The phases-of-treatment model for methadone maintenance: Implementation and evaluation. J Psychoactive Drugs. 1994;26:181-97.
- [Google Scholar]






