Translate this page into:
Clinicopathologic Features and Early Surgical Outcome of Astrocytomas in Eldoret, Kenya
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Astrocytomas are primary central nervous system tumors arising from astrocytes and accounting for up to 37.8% of all brain tumors seen in hospital-based studies from Africa. Despite being common, their patterns and short-term outcomes remain poorly studied in Kenya.
Materials and Methods:
A prospective, descriptive study involving consecutive patients with a histological diagnosis of astrocytoma seen in three hospitals located in Eldoret, Kenya. Clinicopathologic characteristics and outcomes were recorded and patients followed up for 12 weeks.
Results:
Thirty-one patients were recruited over a 1-year period. Majority of them were female (51.6%). Headache (83.9%) and focal neurological deficits (64.5%) were the most common presenting features. Among patients with high-grade tumors, mean duration of illness was 106.03 ± 162.16 days, median functional status was Karnofsky performance status (KPS) score 50, mean tumor size was 110.22 ± 46.16 cm3, and median magnetic resonance imaging (MRI) score was 17. Among patients with low-grade astrocytomas, mean duration of illness was 213.03 ± 344.93 days, median functional status was KPS score 40, mean tumor size was 53.49 ± 54.96 cm3 and median MRI score was 9. Glioblastoma multiforme (GBM) (71%) and diffuse astrocytoma (22.6%) were the predominant histological subtypes. The median Ki-67 proliferative index was 6% for pilocytic astrocytoma, 1.6% for diffuse astrocytoma, and 60% for GBM. Systemic and regional surgical complications occurred in 6.5% and 38.7% of patients, respectively. In-hospital mortality was 19.4% and increased to 25.8% at 12 weeks. The KPS score at discharge was 50 and improved to 60 at 12 weeks. Only 9.7% of patients had acceptable functional status at 12 weeks follow-up.
Conclusions:
In this locality, headache, focal neurological deficits, and reduced functional status are the most common presenting features of astrocytomas while GBM is the most common histological subtype. Tumors are highly proliferative and in the short-term, both surgical and functional outcome are suboptimal.
Keywords
Astrocytoma
brain neoplasms
glioblastoma
Ki67
INTRODUCTION
Astrocytomas are tumors of the central nervous system arising from astrocytes. Epidemiologically, they account for 76–80% of all malignant tumors of glial tissue in population-based studies from high-income countries.[1] Their age-adjusted incidence rate is noted to be slightly higher among males compared to females, and a racial difference reflecting higher rates among Caucasians compared to people of African descent has been reported.[23] In Africa, there is a lack of population-based studies. However, these tumors have been noted to account for 27.9%–37.8% of all brain tumors in hospital-based studies.[45]
There is a paucity of data on the pattern, short-term efficacy (symptomatic relief), and toxicity (adverse events/complications) of therapy among patients with astrocytomas in low and middle-income countries.[6] Within the Kenyan context, astrocytomas constitute the bulk of brain tumors encountered in clinical practice in both adult and pediatric populations.[47] However, their clinical and pathological characteristics as well as therapeutic outcomes remain inadequately reported particularly in the western region of the country. This study aimed to fill this gap.
MATERIALS AND METHODS
Setting and study population
This was a prospective, descriptive census study of both pediatric and adult patients with a confirmed histological diagnosis of astrocytoma seen over a 1-year period. Patients were followed up for a period of 12 weeks after treatment. Patients with recurrent tumors were excluded from the study. The study was carried out in three tertiary hospitals in Eldoret town, in the western part of Kenya. Their catchment area includes western part of Kenya as well as Eastern Uganda and South Sudan. All hospitals had access to a histopathology laboratory as well as magnetic resonance imaging (MRI) facilities.
Study procedure
Eligible patients were sought from neurosurgical clinics and wards in the study hospitals. After obtaining informed consent/assent, a data abstraction form was used to collect data on patient demographics, clinical, MRI, and histological characteristics of brain tumors as well as data on management and outcome. All data were subject to double entry, and access was restricted to the investigators.
Demographic characteristics included age and sex and a note on their referring facility. Clinical characteristics included data on presenting symptoms, duration of illness, and functional status. For all patients, functional status was assessed on recruitment, at discharge from hospital, and at 12 weeks after discharge from hospital. Patients were asked to return for reevaluation. In the event that they were unable to return, they were contacted by telephone. Each patient had an MRI scan which was used to assess tumor size, location, and various other macroscopic features. Axial, coronal, and sagittal T1 and T2-weighted images with contrast enhancement were obtained. As previously described,[8] edema, mass effect, contrast enhancement, borders, signal homogeneity, necrosis, hemorrhage, and flow void were assessed by assigning a score ranging from 1 to 3 depending on whether they were mildly, moderately, or strongly present. A total score (ranging from 8 to 21) was then calculated for each patient.
Data on the choice of therapy, i.e., surgery, radiotherapy, or chemotherapy alone or in combination were also recorded. For those undergoing surgery, data on intraoperative characteristics such as duration of surgery and extent of surgical resection and postoperative outcome such as functional status, regional and systemic complications, length of hospital stay, and perioperative mortality were recorded. The overall presence or absence of both regional and systemic complications were assessed and recorded at the time of discharge by reviewing patient case notes for indicators that any of the complications occurred. Regional complications assessed included operative site hematoma; worsening/new onset seizures, wrong site surgery as well as wound-related complications, i.e., wound dehiscence, cerebral spinal fluid leak, and surgical site infection. Systemic complications such as venous thromboembolism, pneumonia, and renal failure were also assessed.
Following surgery, brain tumor specimens underwent hematoxylin and eosin (H and E) staining. Although tissues were processed in different laboratories, over two thirds of specimens were reviewed by one reference pathologist with good agreement between primary and reference pathologists. The Envision FLEX system (K8000; Dako Denmark A/S) was used for immunostaining. After retrieval, peroxidase blocking was done (100 μL of reagent for up to 5 min) followed by primary antibody staining (100 μL of reagent for up to 20 min). Thereafter, horse-radish peroxidase was applied as the labeled polymer (100 μL of reagent for up to 20 min) followed by substrate working solution (200 μL of DAB + solution for up to 10 min). The process was completed by hematoxylin counterstaining for 5 min. After each stage, washing was done using phosphate-buffered solution. The Ki-67 proliferative index was determined as the number of immunoreactive nuclei per 1000 tumor cells visualized under high power (×40) on the microscope. Photographs of H and E as well as immunohistochemistry slides were taken at low (×10) and high power (×40).
Data analysis
Data on patient demographics, clinical presentation, and pathological (including immunohistochemical) characteristics of the tumors as well as frequency of complications (including mortality) were stratified according to tumor grade and summarized using descriptive statistics on SPSS statistics software v23 (IBM, Armonk, New York, USA).
Ethical consideration and funding
Approval to conduct the study was sought from the Moi University/MTRH Institutional Research and Ethics Committee. Informed consent/assent was sought from eligible patients and confidentiality assured. This study was supported by a science, technology and innovation grant awarded to the investigator by the National Commission for Science, Technology, and Innovation, Kenya.
RESULTS
Sample description
A total of 121 patients with brain tumors were seen during the study. Of these, 31 (25%) had histologically confirmed astrocytoma and were recruited into the study. Overall, their ages ranged from 4 to 73 years with a mean of 44.42 years (standard deviation [SD] 19.14 years). There were 16 female patients (51.6%) and 15 male patients (48.4%). Majority of these patients (64.5%) were referred from Level IV facilities from across western part of Kenya. Table 1 gives a description of the study sample stratified by tumor grade.

Clinical and magnetic resonance imaging features of astrocytomas at presentation
Overall, the mean duration of illness was 137.1 days (SD 229.29 days) while mean time from diagnosis to surgery was 11.65 days (SD 11.09 days). Headache (83.9%) and focal neurological deficits (64.5%) were the most common presenting features. Other features included vomiting (32%), seizures (22.6%), and personality changes (29%). The median Karnofsky performance status (KPS) score at presentation was 50 with only one patient (3.2%) having a nondependent performance status. Majority of tumors involved the cerebrum (90.3%). The average tumor volume was 93.75 cm3 (SD 54.61 cm3). The overall MRI score ranged from 8 to 19 with a median score of 16. Table 2 summarizes these features stratified by tumor grade.

Histological subtypes and proliferative index
Glioblastoma multiforme (GBM) was the most common histological subtype (71%) followed by diffuse astrocytoma (22.6%). Pilocytic astrocytoma occurred in only 6.5% of patients while there were no participants with anaplastic astrocytoma (AA). Out of a total of 31 included patients, 11 did not have Ki-67 immunostaining done owing either to poor tissue processing or preservation. For the remaining 20 patients, the Ki-67 proliferative index ranged from 2% to 10% for pilocytic astrocytoma, 1%–8% for diffuse astrocytoma, and 24%–95% for GBM. Table 3 gives a summary of the pathologic characteristics stratified by tumor grade. The histopathological features and immunohistochemical findings for pilocytic astrocytoma, diffuse astrocytoma and GBM are shown in Figures 1 to 6 respectively.


- Photomicrograph showing pilocytic (Grade I) astrocytoma. Mild cellularity, fibrillar background, and microcysts (H and E, ×40)

- Photomicrograph showing pilocytic (Grade I) astrocytoma. Ki-67 immunostain (×40) showing few immunolabeled nuclei

- Photomicrograph showing diffuse (Grade II) astrocytoma. Moderate cellularity and fibrillar background (H and E, ×40)

- Photomicrograph showing diffuse (Grade II) astrocytoma. Ki-67 immunostain (×40) showing few immunolabeled nuclei

- Photomicrograph showing glioblastoma multiforme (Grade IV astrocytoma). High cellularity, mitoses, and zonal necrosis (H and E, ×40)

- Photomicrograph showing glioblastoma multiforme (Grade IV astrocytoma). Ki-67 immunostain (×40) showing numerous immunolabeled nuclei
Surgical outcome
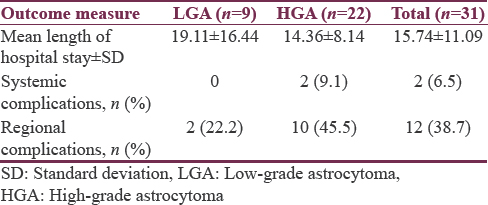
All patients underwent surgery. The mean duration of surgery was 191.13 min (SD 68.75 min). Gross total tumor resection was achieved in 21 (67.7%) patients while 9 (21%) had subtotal resection. One patient underwent biopsy only. Among patients with high-grade tumors, 36.4% were given chemotherapy and 22.7% had radiotherapy by 12 weeks of follow-up. Overall, the average length of hospital stay was 15.74 days (SD 11.09 days). Systemic surgical complications occurred in 2 (6.5%) patients while 12 (38.7%) had regional surgical complications. In the two patients with systemic complications, both developed fever attributed to chest infection while in the intensive care unit. Of the 12 patients with regional complications, 10 had new or worse neurological deficits while 2 patients developed operative site hematoma. Table 4 summarizes surgical outcome stratified by tumor grade.
Functional outcome
The median KPS score at discharge was 50 with 3 patients (9.7%) having nondependent performance status (KPS >80). At 12 weeks follow-up, the median KPS score had improved to 60 with 3 patients (9.7%) having nondependent performance status. Inpatient mortality was 19.4% (6 patients) and by 12 weeks of follow-up mortality stood at 25.8% (8 patients). Table 5 summarized functional outcome stratified by tumor grade.

DISCUSSION
Within the local context, this study is among the first attempts at characterizing astrocytomas and their outcomes. The proportion of brain tumor patients with astrocytomas (25%) is slightly lower than that seen in other studies from Africa.[457] When stratified by tumor grade, the mean age for low-grade astrocytomas (LGAs) was 27.89 years and that for high-grade astrocytomas (HGAs) was 51.18 years while HGAs occurred more frequently in males. These findings are in keeping with other studies.[291011] However, the small sample size and the fact that this was a hospital-based study should be kept in mind. Majority of our patients were referred from Level IV to V facilities from the Western Kenya region. Since the introduction of a devolved system of health governance in Kenya, many Level IV and V facilities have been equipped with computed tomography scan machines and this may account for this observation.
A number of features were assessed on MRI so as to help in arriving at a radiological diagnosis and in planning for treatment. Each feature assessed was scored and an overall score given. Lower overall scores suggest LGAs while higher ones suggest HGAs.[8] The median score for LGAs in this study was 9 while that for HGAs was 17. This is in keeping with what was suggested by Pierallini et al.[8] Although the MRI scores in this study were arrived at using weaker machines than those used in Pierallini's study (0.3T and 0.5T vs. 0.5T and 1.5T), the overall result is similar. Neuroimaging is of particular importance when assessing astrocytic tumors. MRI is useful has when assessing for macroscopic features that cannot be assessed by histology alone, for example, the effect of tumor on surrounding tissues.[812] Further, sampling error during biopsy/tumor resection may lead to underestimation of tumor grade. MRI features suggestive of higher grade tumor may help in arriving at a more accurate grading and help in predicting clinical outcome.
A histological diagnosis was arrived at in each of the recruited patients with GBM and diffuses astrocytoma being the most common histological subtypes. This is in keeping with both hospital- and population-based brain tumor registries in which GBM is among the most common brain tumors.[131314] While the occurrence of the other subtypes is uncommon,[2] AA more frequently but was not encountered in this study perhaps because both AA and GBM exhibit features of poorly differentiated tumor cells making it difficult to tell them apart.[15] Ki-67 immunostaining was performed as a supplement to histopathology. Higher proliferative indices were noted for pilocytic astrocytoma (6%) and glioblastoma multiforme (60%) compared to similar studies.[161718] Such variation may be attributed to differences in fixatives used, antigen retrieval and interpretation methods. However, higher proliferative indices in pilocytic astrocytomas have been noted in other studies and attributed to microglial proliferation although this observation has no impact on long-term prognosis.[16] Tumors with similar histology may have genetic differences that are reflected in variant proliferative indices.[19] It is possible that the higher proliferative indices observed for glioblastoma in this study point to underlying genetic differences that confer worse prognosis in this region. All in all, these findings should be interpreted with caution since this was a small hospital-based study.
Therapy for astrocytic tumors involves surgery which is adequate therapy for LGAs, but more aggressive tumors require MRI surveillance as well as chemoradiation.[215] In this study, all patients underwent surgery, and the majority had gross total tumor resection. Postsurgical therapy includes chemotherapy with temozolomide as well as radiotherapy. According to the National Comprehensive Cancer Network guidelines, the choice of these therapies is determined by the age of the patient, their performance status, and methylguanine methyltransferase (MGMT) promotor status. A majority of the patients with HGA were young and despite low-performance status, they qualified for chemoradiation. However, only 36.4% got it and only 22.7% got radiotherapy within the study follow-up period. Reasons for this are varied. First, access to radiotherapy was a problem since at the time of the study; the nearest radiotherapy machine was in Nairobi (300 km from Eldoret). Second, it is possible that for a majority of patients, the long turnaround time for histology results delayed the starting of chemoradiation. Of note is that MGMT promotor status could not be assessed in our setup and therefore, its impact on choice of postsurgical therapy remains unknown.
In this series, 6.5% of patients experienced systemic complications, and 38.7% experienced regional complications. These figures are higher than those reported in similar studies,[1011] and poor functional status at presentation as well as lack of neuronavigational equipment may explain these findings. However, even in some areas without such equipment, lower complication rates have been reported[6], and other factors may be at play. Majority of the patients with these complications had HGA. However, it is not possible to determine the role of tumor grade in these findings owing to the small sample studied. Tumor size in this study was larger compared to studies in the west.[11] This complicates surgery and predisposes to surgical complications. Overall, in-hospital mortality was 19.4% and this increased to 25.8% at 12 weeks of follow up. Similarly, these figures are higher than those reported in other studies. Further, it is not possible to study the effects of tumor grade on these outcomes owing to the small sample size obtained.
Functional status is as important a presenting feature as it is an outcome.[20] There was no difference in overall KPS score at presentation and at discharge. However, overall functional status had improved by 12 weeks of follow-up. Nonetheless, majority of patients were still dependent with only 9.7% of patients having a score of 80 and above. This observation may be attributed to a number of reasons. First, majority of patients had had their illness for quite some time, and therefore, it is unlikely that a dramatic change in condition would be observed within the time frame available for the study. Indeed, some authors recommend surgery for patients with favorable scores only,[2021] which was not the case in this study. Second, majority of the patients had HGA which is an aggressive and infiltrating tumor with generally poor short- and long-term outcomes.[622]
As a limitation, while this study highlights the pattern of astrocytomas in Eldoret and gives baseline data on their outcomes it did not assess the long-term outcome of astrocytomas, and therefore, the impact of surgical therapy on survival could not be assessed. Further, owing to the small sample size, it was not possible to determine the impact of the various clinical, MRI, and pathological features on outcome.
CONCLUSIONS
In our region, headache, focal neurological deficits, and reduced functional status are the most common presenting features while GBM is the most common histological subtype. The tumors encountered are highly proliferative and in the short-term, both surgical and functional outcome remain suboptimal. However, these are only preliminary results and multicenter research is warranted to confirm these findings, determine the underlying mechanisms for the high proliferative activity of astrocytomas in this region as well as determine the reasons for the observed surgical and functional outcome.
Financial support and sponsorship
This study was supported by a science, technology, and innovation grant awarded to the investigator by the National Commission for Science, Technology and Innovation, Kenya.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We are grateful to all the patients and staff from the study hospitals for their participation in the study. Much gratitude also goes to the managers in the study hospitals for granting permission to conduct the study.
REFERENCES
- CBTRUS. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004-2008. 23 March, 2012 Revision. 2012
- [Google Scholar]
- CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2013;15(Suppl 2):ii1-56.
- [Google Scholar]
- Brain tumours at the Kenyatta National Hospital, Nairobi. East Afr Med J. 2000;77:444-7.
- [Google Scholar]
- Frequency of central nervous system tumors in Delta region, Egypt. Indian J Pathol Microbiol. 2011;54:299-306.
- [Google Scholar]
- Perioperative outcomes following surgery for brain tumors: Objective assessment and risk factor evaluation. J Neurosci Rural Pract. 2012;3:28-35.
- [Google Scholar]
- Posterior cranial fossa tumours in children at Kenyatta National Hospital, Nairobi. East Afr Med J. 2004;81:258-60.
- [Google Scholar]
- Supratentorial diffuse astrocytic tumours: Proposal of an MRI classification. Eur Radiol. 1997;7:395-9.
- [Google Scholar]
- Treatment outcome and prognostic factors of adult glioblastoma multiforme. J Egypt Natl Canc Inst. 2013;25:21-30.
- [Google Scholar]
- Patterns of care and outcome for patients with glioblastoma diagnosed during 2008-2010 in Spain. Neuro Oncol. 2013;15:797-805.
- [Google Scholar]
- Postoperative deterioration in health related quality of life as predictor for survival in patients with glioblastoma: A prospective study. PLoS One. 2011;6:e28592.
- [Google Scholar]
- Central nervous system tumors: Radiologic pathologic correlation and diagnostic approach. J Neurosci Rural Pract. 2015;6:191-7.
- [Google Scholar]
- Current concepts in the pathology and genetics of gliomas. Indian J Cancer. 2009;46:108-19.
- [Google Scholar]
- Practical value of MIB-1 index in predicting behavior of astrocytomas. Indian J Pathol Microbiol. 2011;54:520-5.
- [Google Scholar]
- Use of MIB-1 (Ki-67) immunoreactivity in differentiating grade II and grade III gliomas. J Neuropathol Exp Neurol. 1997;56:857-65.
- [Google Scholar]
- Ki-67 immunostaining in astrocytomas: Association with histopathological grade – A South Indian study. J Neurosci Rural Pract. 2016;7:510-4.
- [Google Scholar]
- Role of ki-67 labeling index as an adjunct to the histopathological diagnosis and grading of astrocytomas. J Cancer Res Ther. 2014;10:641-5.
- [Google Scholar]
- Current trends in the surgical management and treatment of adult glioblastoma. Ann Transl Med. 2015;3:121.
- [Google Scholar]
- Factors involved in maintaining prolonged functional independence following supratentorial glioblastoma resection. Clinical article. J Neurosurg. 2011;114:604-12.
- [Google Scholar]
- Treatment outcomes for patients with glioblastoma multiforme and a low karnofsky performance scale score on presentation to a tertiary care institution. Clinical article. J Neurosurg. 2011;115:220-9.
- [Google Scholar]






