Translate this page into:
Impact of Progression of Parkinson's Disease and Various Other Factors on Generalized Anxiety Disorder
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
While much research has been conducted toward understanding the relationship between prevalence of Parkinson's disease (PD) and generalized anxiety, little has been done considering additional influential factors in the relationship by means of a large ethnically diverse sample. Our study strives to fulfill these deficits in the literature as we set out to determine the impact of progression of PD, age, gender, and Hoehn and Yahr (H and Y) staging of PD on generalized anxiety.
Methods:
A retrospective chart review analysis was performed on PD patients who were regularly examined in a community-based PD and movement disorders center from 2005 to 2010.
Results:
This study consisted of 310 patients with PD among whom 12% had generalized anxiety. Neither age nor gender was significant onset predictors at P = 0.05. The impact of progression of H and Y Stages 2–3 and 2–4 increased the odds of generalized anxiety disorder (GAD) prevalence though it was statistically insignificant at P = 0.05.
Conclusions:
Clinicians should not expect the risk of developing anxiety to depend on gender nor change as a function of age though it may increase with symptomatic progression of PD as outlined by H and Y. To the best of our knowledge, this is the largest and most ethnically diverse prevalence study with a focus on generalized anxiety and PD.
Significant Outcomes and Limitations:
The symptomatic progression of PD, but not age or gender, may be associated with an increased risk for GAD. This study lacked adjustment for potential confounders such as depression and PD medications.
Keywords
Anxiety
gender
Parkinson's disease
Parkinson's disease stage
INTRODUCTION
Parkinson's disease (PD) is a neurodegenerative condition from a loss of dopaminergic neurons, most prominently in the substantia nigra. The main symptoms of PD consist of bradykinesia, resting tremor, rigidity, and postural instability.[1] In addition to these motor symptoms, PD causes many nonmotor symptoms, which in some cases may begin before the motor symptoms. Depression, anxiety, cognitive dysfunction, hallucinations, speech and swallowing problems, bladder dysfunction, constipation, drooling, and autonomic dysfunction are among the nonmotor symptoms of PD.[2] Anxiety disorders, particularly generalized anxiety disorders (GADs), panic disorders, and phobic disorders are reported by a significant number of patients with PD.[1] Anxiety is a pathological state characterized by a feeling of dread accompanied by somatic signs that indicate a hyperactive autonomic nervous system. It is differentiated from fear, which is a response to a known cause.[3] Existing literature indicates that the prevalence of anxiety disorders in patients suffering from PD ranges from 3.6% to 40%,[4] which is higher than in normal populations and other disease comparison populations.[5] Panic disorders are the most common, with prevalence rates ranging from 13% to 30%;[6] one study has even suggested that the prevalence of anxiety disorders may be higher than current estimates, due to certain anxiety disorder characteristics not meeting the conventional Diagnostic and Statistical Manual of Mental Disorders–Fourth Edition (DSM-IV) diagnostic criteria.[7]
Patients with anxiety disorders may complain of having palpitations, dizziness, shortness of breath, episodes of sweating, a feeling of passing out, numbness of lips, and fingertips, in addition to fidgeting and agitation. These episodes are very frightening to the caregiver and may make them feel helpless. A study conducted by Siemers et al. comparing changes in state–trait anxiety inventory (STAI) scores with changes in parkinsonian disability found the magnitude of change in STAI scores correlate with the change in degree of disability due to PD, as well as with disease duration.[8]
About 85% of patients with PD may hyperventilate and experience shortness of breath.[9] It is likely that this shortness of breath is a wearing off symptom in patients with PD.[10] Some studies have shown that patients with PD may have upper airway obstruction, resulting in shortness of breathing, which is reversed by levodopa (LD).[11] Apomorphine (a dopamine agonist) has also been shown to improve respiration in PD patients.[12]
Although anxiety is a known nonmotor symptom of PD, there are many other variables that could affect the prevalence of anxiety in PD.
METHODS
A retrospective chart review analysis of 310 idiopathic PD patients was conducted in a community-based PD and movement disorders clinic. These patients were seen and regularly followed between 2005 and 2010. The Brain Bank criterion was used to diagnose patients with PD.[13] In addition to other nonmotor symptoms, patients in this clinic were routinely screened during follow-up visits for the presence of anxiety symptoms using the DSM-IV diagnostic criteria for GAD.[14] In 2010, the Hoehn and Yahr (H and Y) staging system was used to collect data to determine in which stage of PD was the onset of anxiety in each patient. Patients without anxiety were staged at their final H and Y stage of PD at the point of data collection.
To further examine the prevalence of anxiety, patients were organized according to gender and H and Y stage of PD as presented in Tables 1 and 2, respectively.
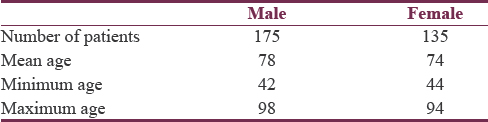

The study protocol confirms to the provisions of the Declaration of Helsinki 1995 (as revised in Tokyo 2004) and has been approved by the Ethics Committee of the Centenary Hospital Division of the Rouge Valley Health System. Each patient gave their informed consent and their case was kept confidential.
The overall age-specific, gender-specific, and PD stage-stratified prevalence rates of anxiety were determined. Logistic regression was performed testing the differences in prevalence between the genders, ages, and PD stages. Analysis of anxiety occurrence with PD stage was conducted with the exclusion of patients in PD Stages 1 and 5 as the number of patients in these stages was too small to allow for their inclusion.
The mean age and mean PD stage of patients with anxiety and those without anxiety were determined.
RESULTS
The overall prevalence of anxiety in this sample of PD patients was 12%. The male- and female-specific prevalence rates were 10.8% and 12.6%, respectively [Table 3]. Table 4 summarizes the PD stage-stratified prevalence rates.
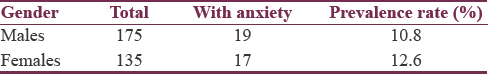
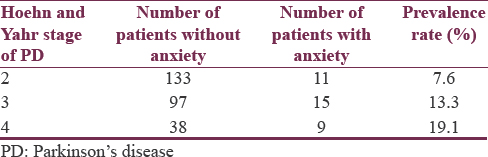
In this statistical analysis, likelihood ratio and Wald statistics indicated that the overall null hypothesis was not significant at 5% level of significance. Although the overall model was not significant, the impact of the progression of the Stages 2–3 and 2–4 increased the likelihood of prevalence of anxiety. The odds of anxiety for Stage 2 are 3.434 times the odds for Stage 4, adjusted for sex and age, with 95% confidence limits from 1.219 to 9.678. The odds of anxiety for Stage 3 are 1.622 times the odds for Stage 4, adjusted for sex and age, with 95% confidence limits from 0.641 to 4.103.
No statistically significant difference was found in the prevalence of anxiety between genders and ages, and no statistically significant difference was found in mean age between patients with anxiety and patients without anxiety.
DISCUSSION
Previous studies examining the relationship between PD and anxiety have produced conflicting results. Although some studies suggest that anxiety in PD patients is related to the factors of age and PD stage, others indicate the lack of a relationship between anxiety and these factors. For instance, a study conducted by Dissanayaka et al. found that the risk of developing anxiety in PD patients decreased with age and increased with PD stage,[4] while another study conducted by Quelhas and Costa found no significant association of anxiety with age and PD stage.[15] The results of our study indicate the lack of a relationship of anxiety with age but demonstrated the increased prevalence of anxiety with an increase of PD stage, in particular, from Stages 2–3 to 2–4. With respect to the relationship between gender and anxiety in patients with PD, most literature indicates no difference between male- and female-specific prevalence rates,[16] with which our findings concur.
This study supports the findings of previous studies of no significant relationship of anxiety with age and gender. However, in contrast, it demonstrates the increased prevalence of anxiety with increasing stage in PD patients, using a relatively large sample of 310 patients as compared to previous studies. Among the studies mentioned previously regarding this relationship, the largest sample size was 79 PD patients in the study by Dissanayaka et al.[4]
In Ontario (the location of this study), approximately 90% of PD patients are over 60 years of age.[17] In this sample, 86% of patients were over 60 years of age. In addition, this sample having 30% more males than females is similar to there being 50% more males than females in the target population.[18] As a result of these factors, it can be concluded with great certainty that this study has a relatively high external validity.
This study also has the advantage of consistently recorded data on all visits of patients with the attending physician. With the data in this study originating from the medical records of only one physician, one can be quite certain that the recording standards and diagnostic criteria of the attending physician remained consistent across all the patients.
To the best of our knowledge, no other study investigating the prevalence of PD and anxiety has been conducted on a large sample size in an ethnically diverse city as Toronto. Indeed, we had various ethnic groups present among our sample of 310 PD patients, including but not limited to Caucasian, Mediterranean, South Asian, South American, Middle Eastern, and Afro-American. Such a large, culturally heterogeneous sample makes our findings highly generalizable.
The major limitation of this study is the lack of adjustment for potential confounders. Two likely confounders of the anxiety–PD relationship are depression and the medications used to treat PD. Depression is a likely confounder of the relationship between PD and anxiety due to many of the DSM-IV criteria for depression being the same for anxiety.[6] This explains the 30%–40% prevalence rate of anxiety in patients with PD being the same as the percent prevalence of depression in these patients. Understandably then, the lifetime risk of developing either depression or anxiety is approximately 60% in such patients.[19] This evidence suggests that depression is a comorbidity associated with anxiety that can confound the relationship between PD and anxiety.
Medications used in the treatment of PD are also a likely confounder of the relationship between anxiety and PD. Among PD medications, LD and dopamine agonists are the most common trigger of anxiety. Furthermore, anxiety may occur during dopamine agonist withdrawal.[20] This usually occurs during both on and off periods, with the majority of patients on LD therapy experiencing these symptoms after several years of the initiation of treatment.[21]
Due to the conflicting evidence in existing literature regarding the relationship of anxiety with age and PD stage, further research regarding this relationship is warranted. In particular, a meta-analysis may be the best approach to move beyond the current status quo of the ambiguity of the relationship of anxiety with age and PD stage.
Regardless of this uncertainty, anxiety, once manifested in patients with PD, needs to be treated. Due to the possible occurrence of anxiety disorders during withdrawal periods from dopamine agonists,[20] as well as during both on and off periods in patients on LD therapy,[21] it would appear unwise to treat anxiety disorders with further dopaminergic medications. Instead, a number of alternative treatments have been observed. Cognitive enhancement intervention along with cognitive behavioral therapy has also been shown to reduce symptoms of anxiety in PD patients. Selective serotonin reuptake inhibitors have also shown effectiveness against anxiety in patients with PD.[22] Deep brain stimulation of the subthalamic nucleus has been shown to decrease anxiety.[23]
CONCLUSIONS
The results of this study suggest that one should not expect the risk of developing anxiety to increase or decrease with increasing age, but as PD stage progresses, the risk of anxiety may increase. Similarly, our results indicate that one should expect no significant difference in the risk of developing anxiety between males and females with PD. To the best of our knowledge, this is the largest and most ethnically diverse prevalence study concerning mainly the association of anxiety and PD.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors acknowledge Hussain Cader, Asim Siddiqi, Rizwan Farid, Ishraq Siddiqi, and Naeem Yusuf, for formatting this report.
REFERENCES
- Pocket Handbook of Clinical Psychiatry. New York City: Williams & Wilkins; 1996.
- Anxiety disorders in Parkinson's disease: Prevalence and risk factors. Mov Disord. 2010;25:838-45.
- [Google Scholar]
- Anxiety rating scales in Parkinson's disease: Critique and recommendations. Mov Disord. 2008;23:2015-25.
- [Google Scholar]
- Prevalence of anxiety disorders and anxiety subtypes in patients with Parkinson's disease. Mov Disord. 2009;24:1333-8.
- [Google Scholar]
- Shortness of breath, a ‘wearing-off’ symptom in Parkinson's disease. Clin Drug Investig. 2009;29:689-91.
- [Google Scholar]
- Reversibility of upper airway obstruction after levodopa therapy in Parkinson's disease. Chest. 1989;96:210-2.
- [Google Scholar]
- Effects of treatment on airway dynamics and respiratory muscle strength in Parkinson's disease. Am Rev Respir Dis. 1993;148:1576-80.
- [Google Scholar]
- Accuracy of clinical diagnosis of idiopathic Parkinson's disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181-4.
- [Google Scholar]
- A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med. 2006;166:1092-7.
- [Google Scholar]
- Anxiety, depression, and quality of life in Parkinson's disease. J Neuropsychiatry Clin Neurosci. 2009;21:413-9.
- [Google Scholar]
- Occurrence of depression and anxiety prior to Parkinson's disease. Parkinsonism Relat Disord. 2010;16:576-81.
- [Google Scholar]
- Current concepts in the diagnosis and management of Parkinson's disease. CMAJ. 2003;168:293-301.
- [Google Scholar]
- Involvement of nitric oxide in Parkinson's disease: Emphasis on sex difference in prevalence. Neurosci Res Commun. 2003;32:135-42.
- [Google Scholar]
- Parkinson disease: Depression and anxiety in Parkinson disease. Nat Rev Neurol. 2010;6:243-5.
- [Google Scholar]
- Dopamine agonist withdrawal syndrome in Parkinson disease. Arch Neurol. 2010;67:58-63.
- [Google Scholar]
- Nonmotor fluctuations in Parkinson's disease: Clinical spectrum and classification. J Neurol Sci. 2010;289:89-92.
- [Google Scholar]
- Acute psychotropic effects of bilateral subthalamic nucleus stimulation and levodopa in Parkinson's disease. Mov Disord. 2003;18:524-30.
- [Google Scholar]






