Translate this page into:
Rare Cranial Nerve Schwannomas: A Retrospective Review of Nontrigeminal, Nonvestibular Cranial Nerve Schwannomas
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Intracranial schwannomas arising from non-trigeminal and non-vestibular sources are extremely rare constituting <0.8% of all schwannomas. In this article, we have analyzed our experience in the management of these rare tumors over a 10-year period.
Material and Methods:
There were a total of 16 cases, with 11 of them undergoing microsurgical resection and 5 undergoing stereotactic radiosurgery (SRS).
Results:
There were no fresh neurological deficit in any of these patients and two patients underwent postoperative SRS for residual tumor. One patient died due to postoperative septicemia.
Conclusion:
Knowledge of these lesions along with their clinicoradiological profile is essential to maintain a high index of suspicion and understand the nuances of treatment.
Keywords
Cranial nerve
microsurgery
rare schwannoma
skull base
INTRODUCTION
Intracranial nerve schwannomas are slow-growing benign tumors that arise from Schwann cells.[1] Most of these (60%) arise from cranial nerves with a preponderance of origin from the sensory nerves. Motor nerves give rise to schwannomas usually in association with neurofibromatosis.[2] Among the nonvestibular schwannomas, trigeminal nerve predominates with an incidence of 0.8%–8% of the total intracranial nerve schwannomas. Other nerve schwannomas are thus exceedingly uncommon and in the descending order of frequency involve the glossopharyngeal, vagal, facial, accessory, hypoglossal, oculomotor, trochlear, and Abducens nerves.[3] Olfactory nerve as an extension of central nervous system (CNS) lacks Schwann cells. Nerve sheath tumors arising from the anterior cranial fossa (ACF) base are uncommon. They usually arise in relation to olfactory groove. Hence, these groove schwannomas are rarely reported.[4] In this series, we report 16 such cases of nonvestibular and nontrigeminal nerve schwannomas managed in our institution over a 10-year period.
MATERIALS AND METHODS
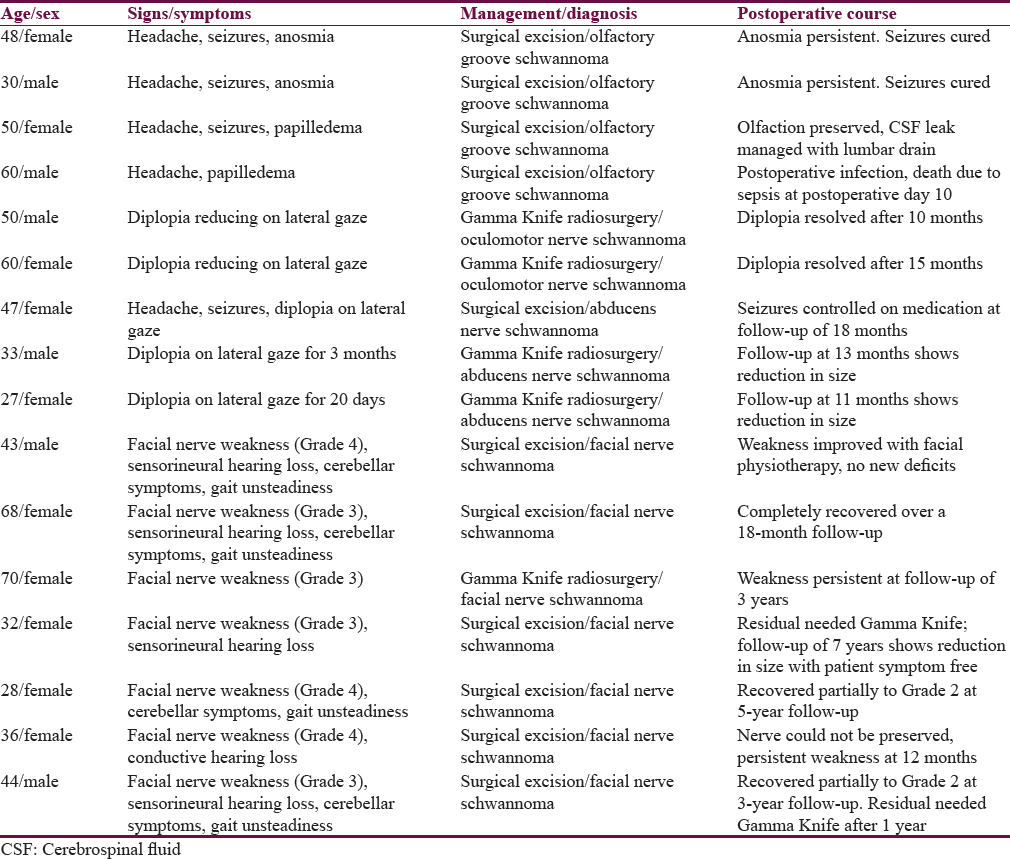
This was a retrospective study of the cases of nonvestibular and nontrigeminal nerve schwannomas managed at the National Institute of Mental Health and Neurosciences, Bengaluru, from 2005 to 2015. Complete demographic, clinicoradiological surgical records were analyzed. Follow-up data were collected from the hospital records. A total of 16 such cases were found [Table 1].
Olfactory nerve schwannomas
There were a total of 4 patients managed for olfactory groove schwannomas [Table 1 and Figure 1]. Clinical features are detailed in Table 1. Histopathology revealed characteristic biphasic pattern of compact Antoni A and loose Antoni B zones with Verocay bodies with positivity for S-100 and vimentin and focal positivity for CD57 and glial fibrillary acidic protein negative, NEW VERSION: Histopathology revealed characteristic biphasic pattern of compact Antoni A and loose Antoni B zones with Verocay bodies. They were also positive for S-100 and vimentin and showed focal positivity for CD57 while being glial fibrillary acidic protein negative, thus differentiating them from a new entity called olfactory ensheathing cell schwannomas which has similar clinicoradiological features but a different cell of origin and thus does not express CD57. Gross total excision was achieved in all of the cases by careful extra-arachnoid dissection with preservation of the olfactory tract [Figure 1]. Olfaction was preserved in the two patients with no previous deficit. One case had postoperative infection with high-grade fever and septicemia and expired on the 10th postoperative day with both blood and cerebrospinal fluid (CSF) culture-growing Klebsiella, which was multidrug resistant. One patient had a transient CSF rhinorrhea, which was managed with a lumbar drain insertion. At follow-up ranging from 6 to 10 years, there has been no recurrence reported.

- (a) Contrast enhanced computed tomography image showing a large heterogenously enhancing lesion in the olfactory groove causing mass effect on both frontal lobes. (b-e) T2 weighted coronal, Fluid attenuation inversion recovery, gradient echo and post gadolinium enhanced T1 weighted Magnetic resonance images showing avid enhancement of olfactory groove lesion with flow voids and perilesional edema. (f) T1 weighted post gadolinium contrast image at follow up showing complete excision of lesion
Oculomotor nerve schwannoma
Two patients with an oculomotor nerve schwannoma presented with progressive diplopia due to III CN paresis [Table 1]. Magnetic resonance imaging (MRI) revealed a tumor, suggestive of a schwannoma, near the brain stem. Both of them underwent stereotactic radiosurgery (SRS) – 12 Gy, 50% isodose line, and 96% coverage. MRI at follow-up (average – 1 year) showed significant reduction in the size of lesion [Figure 2a and b].

- (a) Pre-Gamma Knife magnetic resonance imaging of lesion arising near the interpeduncular cistern from the oculomotor nerve. (b) Post-Gamma Knife scan at 12-month follow-up showing reduction in size of lesion
Abducens nerve schwannoma
There were a total of 3 cases of sixth nerve schwannoma [Table 1]. All of them presented with diplopia on lateral gaze. One patient also had headache and seizures. Radiologically, an isodense lesion on computed tomography (CT) was seen and was hyperintense on T1 and T2 with enhancement on contrast images. One case underwent surgery; subtemporal approach with gross excision of the tumor [Figure 3a and b]. NEW VERSION: One case was approached sub-temporally and underwent gross total excision of tumour [Figure 3a and 3b].

- (a) Preoperative contrast-enhanced magnetic resonance imaging (coronal) showing lesion arising from the left cavernous sinus – abducens nerve schwannoma. (b) Postoperative contrast-enhanced magnetic resonance imaging showing complete excision of lesion
. At follow-up, there were no deficits and no recurrence. The other two patients underwent SRS, and at follow-up at 1-year, the tumor had significantly reduced in size with symptomatic improvement. None of the cases had any features of neurofibromatosis.
Facial nerve schwannomas
A total of seven facial nerve schwannomas were treated. Six of the seven presented as an intrapetrous mass lesion while 1 lesion was seen arising in the pregeniculate region [Table 1 and Figure 4]. All 7 cases presented with sudden onset facial nerve weakness (House–Brackmann Grade III or IV) with the mean duration of symptoms ranging from 6 months to 3 years. Four cases also had associated sensorineural hearing loss of moderate-to-severe grade while 1 patient had conductive type of loss due to possible erosion of the petrous temporal segment. Four cases also had dizziness and unsteadiness of gait with cerebellar signs. All the petrous tumors were >3 cm size and were approached through a middle cranial fossa and transmastoid approach with near gross total tumor resection. The pregeniculate lesion, which was 1.5 cm in size, was subjected to SRS. Nerve preservation was possible in 5 cases, and nerve could not be preserved in 1 case. All patients recovered uneventfully from anesthesia and surgery with no new postoperative deficits. Follow-up was maintained ranging from 12 months to 7 years after surgery (average – 3 years) with no recurrence [Figure 4a and 1b]. However, two patients were subjected to SRS for small residual detected on follow-up scans and have been asymptomatic since.

- (a) Preoperative magnetic resonance imaging showing contrast-enhancing lesion in the right petrous bone eroding the same. (b) Postoperative computed tomography scan showing complete excision of the lesion
DISCUSSION
Nonvestibular, nontrigeminal schwannomas are extremely rare tumors with preoperative diagnosis being difficult due to ambiguous origin and relatively uncharacteristic MRI features.[5] The only clue is the type of presentation and peroperative site of origin of the tumor with careful inspection.
Facial nerve schwannoma
First described by Schmidt in 1930, >300 such cases have been described in literature. The site of origin can be anywhere along the course of the VII CN from the cerebellopontine angle to the extracranial peripheral nerve location.[6] They have been classified based on their site of origin into tympanic, vertical, and the labyrinthine segments. The tympanic is the most common followed by the geniculate segment. Another classification by Sarma et al. divides it into cerebellopontine angle, geniculate, and the tympanomastoid lesions.[7] Clinically, these schwannomas present with facial nerve paralysis or hearing loss and may initially be misdiagnosed as vestibular schwannomas. Hearing deficits may be conductive or sensory/neural, depending on the location of the tumor (i.e., proximal or distal to the geniculate ganglion).[8] CT helps estimate bone damage and planning of surgery. MRI helps evaluate tumor location, size, and extension.[9] Functional tests are necessary to evaluate facial nerve and hearing function. Microsurgery and stereotactic radiosurgery are the therapeutic options. Intraoperative demonstration of the lesion arising from the facial nerve is imperative for the diagnosis and exclusion of other possibilities, especially vestibular schwannomas.[10] Subtemporal, transmastoid, translabyrinthine, and retrosigmoid approaches are the principal routes depending on the site of origin. Preservation of facial nerve function poses the main surgical difficulty. Anatomical nerve conservation, nerve resection with immediate grafting, or delayed hypoglossal-facial nerve anastomosis are the various options. The main predictive factors of postoperative facial function are the degree and duration of facial paralysis before surgery. King and Morrison[11] and Lipkin et al.[7] discouraged early surgery, especially in young patient with no or minimal facial weakness whereas Symon et al.[12] advised a more aggressive approach with reconstruction if required. Hearing loss of sensorineural generally improved after surgery with conductive loss cases being referred for middle ear reconstruction or tympanoplasty as required.
Olfactory groove schwannomas
In 1968, Sturm et al.[13] reported the first case of olfactory groove schwannoma, and since then, >40 such cases have been reported in the literature. Although olfactory nerve in itself lacks Schwann cells, the formation of schwannomas has been explained by two main theories:[14] the developmental hypothesis which suggests transformation of mesenchymal pial cell into ectodermal Schwann cells or migration of the neural crest cells within the substance of the CNS. The nondevelopmental theory states that these schwannomas may arise from Schwann cells of adjacent normal structures such as the perivascular nerve plexus, meningeal branches of the Vth cranial nerve, and anterior ethmoidal nerve innervating the ACF and olfactory groove. In literature, headache (53%) and seizures (41%) were the most common symptoms with anosmia reported in 46% cases.[15] Radiologically, apparent diffusion coefficient mapping, increased diffusion in cystic areas, and occasional restricted diffusion in the solid areas due to high cellularity are seen. On contrast-enhanced images, dural tail was absent. These features helped in differentiating it from meningioma.[16] Total surgical excision done by careful extra-arachnoidal dissection is possible as the tumor is well encapsulated and a plane of cleavage from olfactory tract is often seen thus allowing the preservation of the same. Careful ACF base repair is necessary to prevent the postoperative CSF rhinorrhea.[17]
Abducens nerve schwannoma
It is an exceedingly rare lesion with 21 cases reported to date.[18] In a large series of 63 individual with NF2, sixth nerve schwannoma was found only in 3 cases.[19] According to the anatomy of the sixth nerve, Tung et al.[20] have divided them into 2 types: Type 1: arising from the cavernous sinus leading to a sixth nerve palsy with or without mild headache and Type II: arising mainly in the prepontine area additionally causing signs of increased intracranial pressure. All cases except 1 presented with sixth nerve palsy with hearing impairment being the second most common symptom. In our series, apart from sixth nerve palsy, seizure was the presenting feature in one case. Imaging findings of CN schwannoma usually show a hypodense to isodense lesion on CT scans. On MRI, they are usually hypointense and hyperintense signal on T1-weighted and T2-weighted MRI images, respectively. Cystic changes and a ring enhancement can be seen in some tumors.[20] Intraoperative demonstration of the lesion arising from the abducens nerve is the definitive method for demonstrating the origin. Surgical approaches vary according to the location. For the cavernous sinus and parasellar region lesion, Tung et al.[20] suggested a frontotemporal transcavernous approach, and for lesions in the prepontine region, a simple lateral suboccipital retrosigmoid approach.[21] In our case, the lesion was in the subtemporal region and thus a middle cranial fossa, subtemporal approach was followed. The tumor was peeled off the nerve carefully with preservation of the anatomical continuity.
Third nerve schwannoma
Kovacs[22] in 1927 reported the first case of third nerve schwannoma during an autopsy, and since then, approximately 30 such cases have been reported in the literature.[23] They can be either cisternal, cisternocavernous, or cavernous. The clinical features depend on the location with cisternal schwannomas presenting with isolated CN deficits of the nerve. When these tumors are localized to the cavernous sinus, they can present with either cavernous sinus syndrome or orbital apex syndrome.[23] Gamma Knife radiosurgery is ideal in small tumors while surgery may be necessary in larger ones.
Radiosurgery
The goal of radiosurgery in nonacoustic schwannomas is to achieve tumor control or regression with no new deficits. It consists of mainly two options: radiosurgery or fractionated radiotherapy with proponents on both sides of the spectrum. Huang et al.[24] in their series have demonstrated that nonvestibular schwannomas can be treated with radiosurgery with 9 of the 16 cases having regression and 7 remaining stable in size. In Pollock et al.'s series, on 1 out of the 5 jugular foramen tumors treated experienced regrowth after 7 months.[25] Muthukumar et al.[26] in their series of 18 patients of non-acoustic schwannoma achieved a tumor control rate of 94% with a mean follow-up of 32 months. Our series of 5 cases were treated successfully with none of the cases experiencing any regrowth or any new deficits. Thus, it can be used as treatment arm, especially in tumors located in inaccessible locations where surgery may risk further deficit.
CONCLUSION
Nonvestibular, nontrigeminal schwannomas are very rare tumors of the brain with such a series being rarely reported. Accurate preoperative diagnoses, on the basis of MRI scans, are possible but confirmatory evidence can only be given by intraoperative demonstration of the origin of the lesion. These tumors should be completely excised with current cranial base and microsurgical techniques, with minimal morbidity. Radiosurgery is a tool for the treatment of these lesions, especially when <3 cm in diameter. Recurrence is rare after complete tumor excision and should be the aim during management of these lesions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Glossopharyngeal schwannoma presenting as gagging dysphagia. Postgrad Med J. 1994;70:207-9.
- [Google Scholar]
- The presigmoid petrosal approach. In: Sekhar LN, Oliveira ED, eds. Cranial Microsurgery: Approaches and Techniques. New York: Thieme; 1999. p. :432-63.
- [Google Scholar]
- Trigeminal neurinomas.A series of 111 surgical cases from a single institution. Acta Neurochir (Wien). 1996;138:1027-35.
- [Google Scholar]
- Clinical, radiological, surgical, and pathological determinants of olfactory groove schwannoma. Indian J Neurosurg. 2014;3:86.
- [Google Scholar]
- Intracranial neurilemoma of the spinal accessory nerve. Surg Neurol. 1982;18:18-20.
- [Google Scholar]
- Facial nerve schwannoma: Nerve fibre dissemination. J Laryngol Otol. 1996;110:632-3.
- [Google Scholar]
- Nonvestibular schwannomas of the brain: A 7-year experience. Neurosurgery. 2002;50:437-48.
- [Google Scholar]
- Intracranial facial nerve neurinoma: Surgical strategy of tumor removal and functional reconstruction. Surg Neurol. 1998;49:538-46.
- [Google Scholar]
- Localized mastoiditis simulating a facial nerve schwannoma on MRI. J Laryngol Otol. 1994;108:1008-9.
- [Google Scholar]
- The puzzling olfactory groove schwannoma: A systematic review. Skull Base. 2011;21:31-6.
- [Google Scholar]
- Solitary olfactory schwannoma without olfactory dysfunction: A new case report and literature review. Neurol Sci. 2012;33:137-42.
- [Google Scholar]
- Intratumoral microhemorrhages on T2*-weighted gradient-echo imaging helps differentiate vestibular schwannoma from meningioma. AJNR Am J Neuroradiol. 2008;29:552-7.
- [Google Scholar]
- Usefulness of T2*-weighted MR sequence for the diagnosis of subfrontal schwannoma. J Neuroradiol. 2007;34:330-3.
- [Google Scholar]
- Dumbbell-shaped abducens schwannoma: Case report. Neurol Med Chir (Tokyo). 2014;54:331-6.
- [Google Scholar]
- Neurinoma of the third, fourth, and sixth cranial nerves: A survey and report of a new fourth nerve case. Surg Neurol. 1992;38:216-24.
- [Google Scholar]
- A case of abducens neurinoma mimicking acoustic neurinoma. Comput Med Imaging Graph. 1998;22:257-61.
- [Google Scholar]
- About a solitary neurinoma of the oculomotor nerve. Zentralbl Allg Pathol. 1927;40:518-22.
- [Google Scholar]
- Schwannoma in patients with isolated unilateral trochlear nerve palsy. Am J Ophthalmol. 1999;127:183-8.
- [Google Scholar]
- Preservation of cranial nerve function after radiosurgery for nonacoustic schwannomas. Neurosurgery. 1993;33:597-601.
- [Google Scholar]
- Stereotactic radiosurgery for jugular foramen schwannomas. Surg Neurol. 1999;52:172-9.
- [Google Scholar]






