Translate this page into:
Recurrent Glioblastomas Exhibit Higher Expression of Biomarkers with Stem-like Properties
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Despite advances in the treatment of glioblastoma (GBM), the prognosis of patients continues to remain dismal. This unfavorable prognosis is mainly attributed to the tumor's propensity for progression and recurrence, which in turn is due to the highly aggressive nature of the persisting GBM cells that actively egress from the main tumor mass into the surrounding normal brain tissue. Such a recurrent tumor described to have a more malignant potential is highly invasive and resistant to current therapies, probably due to increased stemness and preferential selection of therapy-resistant clones of tumor cells. However, there is a paucity of literature on the expression of biomarkers in the recurrent GBM tumors that could have a role in conferring this aggressiveness.
Aim:
To identify the differences in the expression pattern of selected biomarkers in paired tissue samples of GBM.
Material and Methods:
A retrospective study on 30 paired samples of GBM (newly diagnosed/primary and recurrent) archived in the Department of Neuropathology, NIMHANS (2006–2009), was carried out. After obtaining clinical and demographic details, tumors were characterized histomorphologically and immunohistochemically on formalin-fixed paraffin-embedded tissues with reference to expression of biomarkers such as p53, epidermal growth factor receptor (EGFR), insulin-like growth factor binding protein 3 (IGFBP-3), sex determining region Y-box 2 (SOX2), and topoisomerase 2 A (Top2A). The results were statistically analyzed.
Results:
It was observed that while p53 and IGFBP-3 expression remained unaltered in paired samples, a significant increase in the expression of EGFR (P < 0.01) was noted in the recurrent tumors. Among the other biomarkers, SOX2 expression was higher in the recurrent tumors when compared to the primary tumors (P < 0.01). Conversely, the expression of Top2A was reduced in recurrent tumors (P = 0.05). Mild elevation in the expression of IGFBP-3 was observed in recurrent tumors but was not statistically significant.
Conclusion:
A significant increase in the expression of SOX2 in recurrent tumors probably indicates the presence of undifferentiated cells with stem-like properties in these tumors. EGFR is known to mediate SOX2 expression thereby resulting in stemness of the glioma cancer cells, which could further explain its overexpression in recurrent GBMs. Furthermore, a decreased expression of TOP2A observed in the recurrent tumors could probably be due to reduction in chemosensitivity to temozolomide, which has been shown in earlier studies. We also noted that p53 expression remained unaltered in the recurrent tumors when compared to the primary, suggesting the absence of preferential clonal expansion of p53 mutant cells following exposure to radiochemotherapy. Our study reiterates the fact that GBM recurrences are associated with molecular alterations that probably contribute to radiochemoresistance, increased invasiveness, therapeutic efficacy, and stemness.
Keywords
Epidermal growth factor receptor
glioblastoma
recurrence
sex determining region Y-box 2
topoisomerase 2 A
INTRODUCTION
Glioblastoma (GBM) is the most frequent and aggressive malignant primary brain tumor in adults. The neoplasm exhibits a significant intratumoral heterogeneity at the cytopathological, transcriptional, and genomic levels.[1] GBM harbors a plethora of cytological and molecular alterations and there are a discrete number of genetic and signaling pathway events that appear to be central to GBM pathogenesis and survival. The current standard of care for GBM patients includes surgical resection followed by chemoirradiation.[2] Despite advances in the treatment of GBM, the prognosis continues to remain dismal.[3] The tumors invariably relapse, and treatment options are limited when this occurs. The guidelines for the management of recurrent GBMs are not well established.[4]
This unfavorable prognosis of GBM is mainly due to its high propensity for progression and inevitable recurrence and due to the aggressive behavior of the persisting GBM cells that actively egress from the main tumor mass into the surrounding normal brain tissue. Such a recurrent tumor has been described to be more aggressive, invasive, and more resistant to current therapies. The reason for this has been postulated to be due to increased stemness along with preferential selection of therapy-resistant clones of tumor cells.[5] GBM is one of the most intensively investigated human malignancies. The heterogeneity of GBM at its cellular, molecular, and genetic levels is more pronounced in the recurrent setting making it one of the most complex tumors being studied and thus hindering the identification of potential targets.[67] The current knowledge and literature regarding the molecular-pathogenetic changes in the recurrent GBMs, especially on paired samples, are limited.[67891011]
Our group had previously studied the role of well-established markers such as p53 and epidermal growth factor receptor (EGFR), as well as novel biomarkers, such as the insulin-like growth factor binding proteins (IGFBP-2, 3, and 5), and established their prognostic significance in newly diagnosed GBM.[12] The functional role of IGFBPs was also studied.[13] Furthermore, our team had shown that topoisomerase 2 A (TOP2A) transcript levels determine the chemosensitivity of GBM to temozolomide therapy and that increased TOP2A levels improved survival in GBM patients receiving temozolomide chemotherapy.[14] Since the role of these biomarkers is not known in the recurrent settings, in the present study, we aimed at evaluating and identifying the differences in the expression pattern of biomarkers comprising p53, EGFR, IGFBP-3, sex determining region Y-box 2 (SOX2), and Top2 A in paired tissue (newly diagnosed and recurrent) samples of GBM. Knowledge of biomarker expression of matched sets of primary and recurrent GBM may shed light on the molecular heterogeneity and the temporal sequence of molecular alterations that arise in response to the selective pressures of radiation and/or chemotherapy in the recurrent setting.
MATERIALS AND METHODS
This is a retrospective study performed on archived formalin-fixed paraffin-embedded (FFPE) sections of 30 paired samples of GBM (newly diagnosed/primary and recurrent) diagnosed and operated between 2006 and 2009. All the patients before re-surgery had received standard radiation therapy with concomitant followed by cyclical chemotherapy using temozolomide.[12] These patients represented a subset of the cohort evaluated in the previous study by our team.[12] The patients were followed up and recurrences were noted. After obtaining relevant clinical and demographic details from the case records, histomorphological features were reviewed using routine hematoxylin and eosin stain. Immunohistochemistry (IHC) was performed on archival FFPE sections of paired samples of GBM to detect expression of biomarkers: (a) p53, (b) EGFR, (c) IGFBP-3, (d) SOX2, and (e) Top2A. FFPE blocks were sectioned at 3–4 μm, deparaffinized, and rehydrated. Briefly, after the initial processing steps, 100–200 μl of primary antibody was added to each slide and incubated for overnight at room temperature. For EGFR, rabbit polyclonal antibody from BIOGENEX (1:25 dilution), for IGFBP3 rabbit polyclonal antibody from Santa Cruz (1:50 dilution), for SOX2 rabbit monoclonal antibody from Cell signaling (1:100 dilution) were used, whereas in case of p53, mouse monoclonal antibody from Biocare, (1:100 dilution) and for Top 2A mouse monoclonal antibody from Dako (1:70 dilution) were used. The sections were incubated with enhancer for 30’ (BIOGENEX, Santa Cruz). Supersensitive™ poly-HRP Secondary Antibody Kit from BIOGENEX and Santa Cruz was used. Labeled antigens were visualized by application of 3’,3-diaminobenzidine as the chromogen for 10 min. Scoring of the biomarkers was done according to the intensity and extent of the IHC staining of tumor cells on the basis of manual interpretation using a Binocular Microscope (Olympus, BX 53). Negative controls were treated identically except that the primary antibody was omitted. A visual semiquantitative grading scale was applied to assess the intensity of the immunoreactivity in a manner similar to our earlier studies.[121314] The assessment was as follows: zero (0) if the staining was absent, 1+ if it was weak, and 2+ if it was strong. Only 2+ staining intensity was considered for analysis. The cytoplasmic and nuclear positivity were scored separately. The labeling index was expressed as a percentage of cells that showed 2+ positive staining among the total number of cells that were counted. A minimum of 2000 cells were counted. To evaluate the difference in the extent of expression of various biomarkers in the initial and recurrent GBM samples, paired t-test was employed. A P < 0.05 was considered to be statistically significant.
RESULTS
A total of sixty samples (30 pairs) were included in the study and evaluated. The median age was 48.35 years (age range: 30–77 years) with a male: female ratio of 1.4:1. The recurrence period ranged from 3 months to 29 months. Nuclear staining was noted for p53, SOX2, and Top2A, while IGFBP-3 revealed both cytoplasmic and nuclear staining and EGFR showed membrane cytoplasmic staining. We observed an increase in EGFR and SOX2 expression in recurrent GBM tumors when compared to the primary tumors. There was no observable change in the expression pattern of IGFBP-3 in the paired GBM samples. Furthermore, we noted that the extent of p53 expression remained identical in both initial and recurrent GBM samples. Interestingly, there was a reduction of Top2A expression in the recurrent tumors. The results and the statistics are indicated in Table 1 and Figure 1. The representative IHC staining images of these biomarkers in the initial and recurrent GBM tumors are depicted in Figure 2a-g.
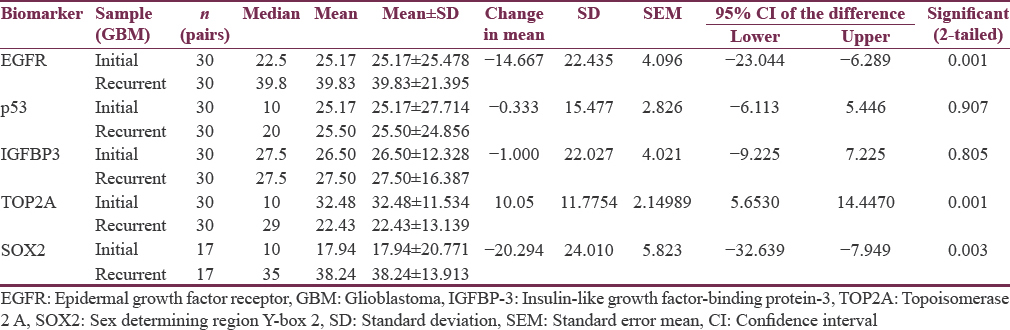
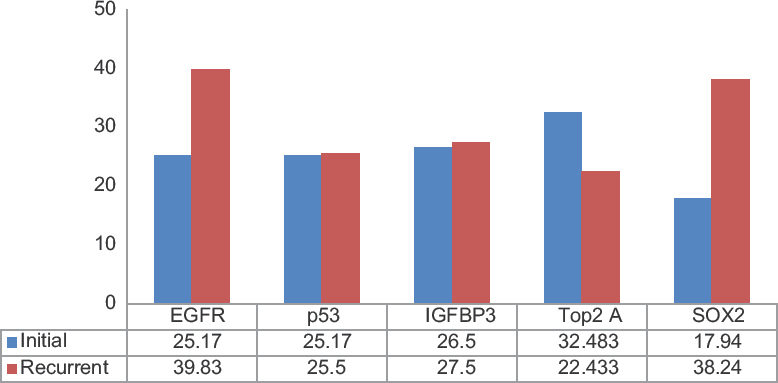
- Bar chart showing extent of expression of biomarkers (mean labeling index values) in paired samples
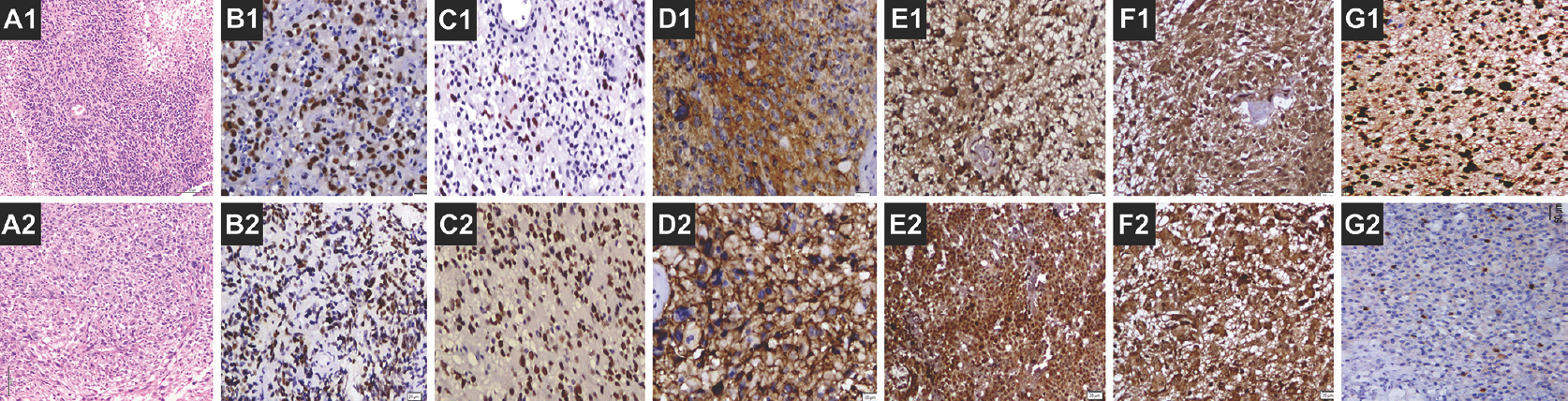
- Microphotograph showing glioblastoma on initial diagnosis – A1 (H and E, ×50), B1 (p53, ×100), C1 (SOX2, ×100), D1 (EGFR, ×200), E1 and F1 (IGFBP-3, ×100), and G1 (Top2A, ×100). Microphotograph showing glioblastoma on recurrence – A2 (H and E, ×50), B2 (p53, ×100), C2 (SOX2, ×100), D1 (EGFR, ×50), E2 and F2 (IGFBP-3, ×100 and ×200, respectively), and G2 (Top2A, ×100)
DISCUSSION
GBM is the most common malignant brain tumor in adults.[15] GBM almost always recurs even after the currently available multimodal therapy. The median time to disease recurrence is 6.9 months with >90% of GBM tumors recurring at the original site.[16] In the present study, the recurrence was at the original site in all the cases. Recurrent GBMs are well documented to be more invasive, aggressive, and less susceptible to radiochemotherapy. The biological profile of recurrent tumors has been shown to be different from its counterpart newly diagnosed GBM.[17] Mechanisms of GBM progression and resistance to radiochemotherapy remain incompletely understood. Keeping in mind the effect of radiochemotherapy on preferential elimination of the susceptible population of tumor cells, the current study evaluated the expression of biomarkers which included markers representing tumor suppressor gene protein (p53), growth factor receptors (proto-oncogene-EGFR), invasion (IGFBP-3), stem cell (SOX2), and proliferation (TOP2A) biomarkers in paired samples of GBM from a cohort of 30 patients who underwent uniform postsurgical radiochemotherapy.
Mutations of Tp53 gene are detected in 65% of secondary and 28% of primary GBMs.[18] Although p53 expression is only an indicator and not concrete proof of mutation in the Tp53 gene, it is often used as a surrogate marker for Tp53 mutations. While p53 expression has been an early feature in low-grade gliomas, it was noted that such population of p53 expressing cells shows a preferential clonal expansion with increasing grades of the tumor. However, such a phenomenon in GBMs is not known.[19] We did not observe a change in the expression of p53 in paired samples, indicating that the p53 expression remains unaltered at recurrence. This probably suggests the absence of a preferential clonal expansion of p53 mutant cells, following exposure to radiochemotherapy. Our observation is supported by an earlier study by Hulsebos et al., who analyzed 12 match paired GBMs and found new LOH at chromosome regions 1p36, 19q13, 10q23, and 1q25 in recurrent tumors, but new Tp53 mutations were not observed.[6] Deininger et al. described a correlation between a high p53 score (detected by the mAb DO-1 which detects wild-type as well as mutated p53) and a short time to tumor progression only in those patients who underwent radiochemotherapy. They also found that radiation treatment led to a reduction in p53 scores (detected by the mAb DO-7 which detects mainly mutated p53 protein) and an increase in bcl-2 scores.[20]
EGFR overexpression is detected in nearly 50% of cases, including over two-thirds of those cases presenting as de novo primary GBMs.[21] The current study demonstrates a significant increase in the expression of EGFR protein at tumor recurrence suggesting a clonal expansion of the EGFR expressing cells following radiochemotherapy. EGFR expression has been shown to be induced by radiation and plays a key role in repair of radiation-induced DNA damage.[2223] Our findings are in contrast with a previous study where the authors noted a decrease in expression of both EGFR and p53 (wild type) in the recurrent sample.[24] The data from van den Bent et al.’ study showed that, in spite of some quantitative differences, the EGFR status remained stable in the majority (84%) of tumors evaluated.[25] Stark et al. compared the IHC expression of p53, mdm2, EGFR, and msh2 in initial GBM with that of recurrent lesions and found recurrent GBM exhibited reduced p53/mdm2/EGFR/msh2 expression, in contrast to our findings on p53 and EGFR. However, neither p53/mdm2/EGFR/msh2 expression nor reduced expression in recurrent GBM was associated with survival.[26]
IGFBP-3 has recently been demonstrated as a GBM-specific biomarker.[12] The expression of IGFBP-3 has been shown to contribute to aggressiveness by bringing about increased proliferation, migration, and invasion.[1227] There was a mild increase in the expression of IGFBP-3 in the recurrent tumors; however, the difference was not statistically significant. This probably suggests a possible redundancy of these proteins with respect to their functional role in a recurrent setting.
SOX2 is considered as a stem cell marker that has been shown to be overexpressed in GBM and associated with proliferation and tumorigenicity.[28] The transcriptional factor SOX2 and EGFR-mediated signaling are both required for self-renewal of neural precursor cells (NPCs). However, the mechanism by which these factors coordinate and regulate this process is largely unknown. The maintenance of stemness is thought to be associated with poor outcome in GBM.[29] In our study, we noted that the median expression level of SOX2 was significantly higher in the recurrent samples suggesting increased stemness in the recurrent GBM tumor. Increase in SOX2 expressing cells in the recurrent tumor could be either due to an effect of radiation or chemotherapy-induced stemness or preferential survival of glioma cells with stem-like property. These initial findings could be further validated in larger cohorts since SOX2 could be a potential molecule for the development of targeted therapy in GBM.[28303132]
DNA topoisomerase 2 A (TOP2A) is one of the important isoforms which affects the topological structure of DNA by interacting with the double-helix DNA, thus playing an important role in DNA replication, transcription, recombination, condensation, and segregation.[3334] In glioma, high levels of TOP2A mRNA and protein have been noted in GBMs in comparison with grade II and III astrocytomas.[3536] Through functional studies, Arivazhagan et al. described the possible mechanism of better response of GBM with TOP2A overexpression to temozolomide chemotherapy.[35] They observed that temozolomide inhibits TOP2A activity in vitro and also demonstrated that TOP2A levels of glioma cells determine their sensitivity to temozolomide, with cells becoming resistant to temozolomide upon downregulation of TOP2A.[35] In breast cancers, it has been reported that amplification of TOP2A may account for chemosensitivity to anthracycline therapy.[37] Further, in vitro studies using different experimental methods have previously proved that sensitivity to the TOP2A inhibitors depends on the level of expression of TOP2A in cancer cells, that is, cells with a low concentration of TOP2A protein are less sensitive to TOP2A-inhibiting drugs than cells containing a high concentration of TOP2A.[37383940] In the present study, significant reduction in the expression of TOP2A indicates inhibition of its expression following temozolomide therapy. Therefore, it can be extrapolated that recurrent GBM tumors probably would not respond to re-challenge with temozolomide treatment. In fact, decreased expression of TOP2A is probably an important mechanism of resistance to several chemotherapeutic agents.
The development of targeted molecular therapy for managing recurrent GBMs in particular presents new opportunities, as well as new challenges. It will primarily require a validation of the expression pattern of targetable molecules in the recurrent GBM tumor tissues. To accomplish this, we will need to develop an expanded understanding of the molecular and genetic changes in paired samples of GBM, which could be obtained through approaches such as gene expression profiling and proteomic studies coupled with careful biological validation and the development of new clinical markers.
CONCLUSION
The present study identifies a set of biomarkers with altered expression in the recurrent tumor (in comparison to the newly diagnosed GBM at its primary setting) suggesting the possible role played by these molecular alterations contributing to radiochemoresistance, increased invasiveness and stemness, resulting in tumor recurrence. Overexpression of EGFR and SOX2 indicates persistence and proliferation of undifferentiated cells with stem-like properties in the recurrent tumor, since EGFR is known to mediate SOX2 expression resulting in stemness of the glioma cancer cells. Decreased expression of TOP2A probably indicates chemoresistance to temozolomide in recurrent setting. Recurrent GBM develops from differing NPCs/differing molecular drivers and are more heterogeneous with varying treatment responses. Identification of molecular features associated with recurrences would aid in better understanding of the pathogenetic process and might provide clues for the development of efficient treatments that can specifically target of glioma cancer stem cells.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97-109.
- [Google Scholar]
- Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987-96.
- [Google Scholar]
- Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J Clin Oncol. 2002;20:1375-82.
- [Google Scholar]
- Standards of care for treatment of recurrent glioblastoma – Are we there yet? Neuro Oncol. 2013;15:4-27.
- [Google Scholar]
- Cellular plasticity regulated cancer stem cell niche: A possible new mechanism of chemoresistance. Cancer Cell Microenviron. 2014;1:pii:e295.
- [Google Scholar]
- Molecular-genetic characterisation of gliomas that recur as same grade or higher grade tumours. J Neurol Neurosurg Psychiatry. 2004;75:723-6.
- [Google Scholar]
- Abnormalities of p16, p15 and CDK4 genes in recurrent malignant astrocytomas. Oncogene. 1996;13:661-4.
- [Google Scholar]
- Different molecular patterns in glioblastoma multiforme subtypes upon recurrence. J Neurooncol. 2010;96:321-9.
- [Google Scholar]
- BCL-2 family protein expression in initial and recurrent glioblastomas: Modulation by radiochemotherapy. J Neurol Neurosurg Psychiatry. 1999;67:763-8.
- [Google Scholar]
- VEGFR2 expression and TGF-β signaling in initial and recurrent high-grade human glioma. Oncology. 2011;81:126-34.
- [Google Scholar]
- Grade-specific expression of insulin-like growth factor-binding proteins-2, -3, and -5 in astrocytomas: IGFBP-3 emerges as a strong predictor of survival in patients with newly diagnosed glioblastoma. Cancer Epidemiol Biomarkers Prev. 2010;19:1399-408.
- [Google Scholar]
- Insulin like growth factor binding protein 4 promotes GBM progression and regulates key factors involved in EMT and invasion. J Neurooncol. 2014;116:455-64.
- [Google Scholar]
- P53 stratification reveals the prognostic utility of matrix metalloproteinase-9 protein expression in glioblastoma. Neurol India. 2015;63:399-404.
- [Google Scholar]
- Emerging insights into the molecular and cellular basis of glioblastoma. Genes Dev. 2012;26:756-84.
- [Google Scholar]
- Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343:189-93.
- [Google Scholar]
- Revisiting the role of p53 in primary and secondary glioblastomas. Anticancer Res. 2006;26:4633-9.
- [Google Scholar]
- P53 immunoexpression as a prognostic marker for human astrocytomas: A meta-analysis and review of the literature. J Neurooncol. 2010;100:363-71.
- [Google Scholar]
- Distinct radiochemotherapy protocols differentially influence cellular proliferation and expression of p53 and bcl-2 in glioblastoma multiforme relapses in vivo . J Neurooncol. 2000;48:121-9.
- [Google Scholar]
- Nuclear signaling of EGFR and EGFRvIII in glioblastoma. In: Garami M, ed. Molecular Targets of CNS Tumors. Croatia: InTech; 2011. p. :515-36.
- [Google Scholar]
- EGFR overexpression and radiation response in glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2001;51:410-8.
- [Google Scholar]
- Radiation-induced EGFR-signaling and control of DNA-damage repair. Int J Radiat Biol. 2007;83:781-91.
- [Google Scholar]
- Amplification of epidermal growth factor receptor gene in gliomas: Histopathology and prognosis. J Neuropathol Exp Neurol. 1992;51:84-90.
- [Google Scholar]
- Changes in the EGFR amplification and EGFRvIII expression between paired primary and recurrent glioblastomas. Neuro Oncol. 2015;17:935-41.
- [Google Scholar]
- P53, mdm2, EGFR, and msh2 expression in paired initial and recurrent glioblastoma multiforme. J Neurol Neurosurg Psychiatry. 2003;74:779-83.
- [Google Scholar]
- STAT-1 expression is regulated by IGFBP-3 in malignant glioma cells and is a strong predictor of poor survival in patients with glioblastoma. J Neurosurg. 2014;121:374-83.
- [Google Scholar]
- SOX2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity. Stem Cells. 2009;27:40-8.
- [Google Scholar]
- A mesenchymal glioma stem cell profile is related to clinical outcome. Oncogenesis. 2014;3:e91.
- [Google Scholar]
- Conversion of differentiated cancer cells into cancer stem-like cells in a glioblastoma model after primary chemotherapy. Cell Death Differ. 2014;21:1119-31.
- [Google Scholar]
- Sox2 is required to maintain cancer stem cells in a mouse model of high-grade oligodendroglioma. Cancer Res. 2014;74:1833-44.
- [Google Scholar]
- Mir-21-sox2 axis delineates glioblastoma subtypes with prognostic impact. J Neurosci. 2015;35:15097-112.
- [Google Scholar]
- Structure and function of type II DNA topoisomerases. Biochem J. 1994;303(Pt 3):681-95.
- [Google Scholar]
- Higher topoisomerase 2 alpha gene transcript levels predict better prognosis in GBM patients receiving temozolomide chemotherapy: Identification of temozolomide as a TOP2A inhibitor. J Neurooncol. 2012;107:289-97.
- [Google Scholar]
- Quantitative analysis of topoisomerase IIalpha to rapidly evaluate cell proliferation in brain tumors. Biochem Biophys Res Commun. 2005;331:971-6.
- [Google Scholar]
- HER-2/neu and topoisomerase IIalpha in breast cancer. Breast Cancer Res Treat. 2003;78:299-311.
- [Google Scholar]
- Transfection of human topoisomerase II alpha into etoposide-resistant cells: Transient increase in sensitivity followed by down-regulation of the endogenous gene. Biochem J. 1996;319(Pt 1):307-13.
- [Google Scholar]
- Isolation of genetic suppressor elements, inducing resistance to topoisomerase II-interactive cytotoxic drugs, from human topoisomerase II cDNA. Proc Natl Acad Sci U S A. 1993;90:3231-5.
- [Google Scholar]
- Ectopic expression of inactive forms of yeast DNA topoisomerase II confers resistance to the anti-tumour drug, etoposide. Br J Cancer. 1996;73:1201-9.
- [Google Scholar]






