Translate this page into:
Extensive Restricted Diffusion in Bilateral Hemispheric White Matter Following Diffuse Hypoxic Injury Due to Hanging
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
A 56-year-old woman, with alleged history of hanging 10 days back, was rescued and taken to a local hospital. She was unconscious, intubated, and managed conservatively. At the time of admission to our hospital, her Glasgow Coma Scale was E2VtM1, with quadriplegia, bilateral plantar reflex showing extensor response. Pupils were equal bilaterally, measuring 2 mm and responding to light. Initial magnetic resonance imaging (MRI) of the brain performed at an outside center [Figure 1] was reported as normal. Repeat MRI of the brain obtained in our institute showed abnormal T2/fluid-attenuated inversion recovery (FLAIR) hyperintensity of bilateral caudate nucleus and putamen, multiple cortices in bilateral temporo-parieto-occipital lobes [Figure 2a–c], with effaced adjacent sulci. Subtle abnormal T2/FLAIR hyperintensity was seen in corpus callosum [Figure 2c], bilateral temporooccipital and parietal lobe white matter, with preservation of normal white matter in frontal lobes [Figure 2a and b]. Diffusion-weighted imaging images showed abnormal hyperintensity in bilateral temporooccipital and parietal lobe white matter and corpus callosum [Figure 2d], corresponding region hypointensity in apparent diffusion coefficient [Figure 2e and f], suggestive of diffusion restriction. Based on MRI images, severe hypoxic brain injury was diagnosed.
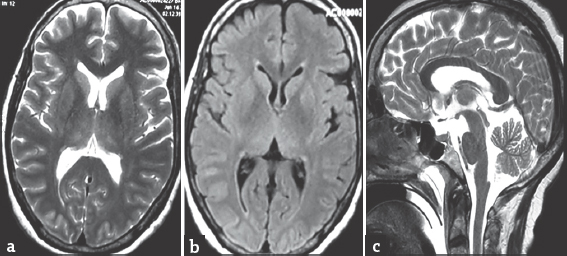
- (a and b) Axial T2 and fluid-attenuated inversion recovery magnetic resonance images showing normal gray and white matter, basal ganglia, and thalami, (c) sagittal T2 image showing normal signal intensity in corpus callosum

- (a and b) Axial fluid attenuated inversion recovery and T2-weighted magnetic resonance images during follow-up showing gyriform abnormal hyperintensity sparing frontal lobe and basal ganglia, subtle T2/fluid-attenuated inversion recovery hyperintensity of white matter, (c) sagittal T2-weighted image showing abnormal T2 hyperintensity of corpus callosum, (d-f) axial diffusion-weighted imaging and apparent diffusion coefficient images showing diffusion restriction
Serial changes are seen in conventional MRI in Wallerian degeneration. No abnormal white matter T2 signal intensity changes will be seen during initial 4 weeks, T2-weighted/FLAIR hypointensity seen during 4–10 weeks, and hyperintensity from 10 to 12 weeks onward.[1] Initial normal T2/FLAIR hyperintensity is due to intact distal axons, T2/FLAIR hypointensity during second phase is due to disintegration of axons and myelin along with alteration in protein-lipid-water ratio, while in late stage, T2/FLAIR hyperintensity is to gliosis. Adult diffuse hypoxic injury of prolonged duration causes extensive cortical damage, producing gyriform diffusion restriction in acute stage. Wallerian degeneration producing diffusion restrictions in subacute stage limited to projection fibers (pontocerebellar fibers,[2] corticospinal tract,[3] or subcortical U-fibers only)[4] is described. Few atypical MRI findings of adult diffuse hypoxic injury such as (i) sparing of posterior circulation,[5] (ii) only basal ganglia involvement, (iii) selective occipital and perirolandic subcortical white matter involvement, (iv) motor cortex involvement, and (v) basal ganglia and visual cortex involvement[6] are also described. In the present case, extensive diffusion restriction and T2/FLAIR hyperintensity were noted involving cortex and white matter, with peculiar sparing of frontal lobe gray and white matter. The pathomechanism behind sparing of frontal lobe remains elusive.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Wallerian degeneration after cerebral infarction: Evaluation with sequential MR imaging. Radiology. 1989;172:179-82.
- [Google Scholar]
- Restricted diffusion in Wallerian degeneration of the middle cerebellar peduncles following pontine infarction. Pol J Radiol. 2010;75:38-43.
- [Google Scholar]
- Diffusion-weighted imaging of acute corticospinal tract injury preceding Wallerian degeneration in the maturing human brain. AJNR Am J Neuroradiol. 2003;24:1057-66.
- [Google Scholar]
- Iowa City/US, Iowa City, IA/US. Intramyelinic edema on diffusion-weighted imaging correlated with pathology and pathophysiology. European congress of Radiology 2013, Poster no 2013/C-2139
- [Google Scholar]
- Hypoxic-ischemic brain injury: Imaging findings from birth to adulthood. Radiographics. 2008;28:417-39.
- [Google Scholar]





