Translate this page into:
Toxic effect of khat (Catha edulis) on memory: Systematic review and meta-analysis
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
People use khat (Catha edulis) for its pleasant stimulant effect of physical activity, consciousness, motor, and mental functions. Although there are reports assessing the effect of khat on memory, there was no study based on formal systematic review and meta-analysis.
Objective:
We have therefore conducted this meta-analysis to determine the level of evidence for the effect of khat (C. edulis Forsk) on memory discrepancy.
Methods:
MEDLINE, Cochrane Library, PubMed, Academic Search Complete, SPORTDiscus, ScienceDirect, Scopus, Web of Science, and Google Scholar were searched to retrieve the papers for this review. Keywords utilized across database search were khat, cat, chat, long-term memory, short-term memory, memory deficit, randomized control trial, and cross-sectional survey. The search was limited to studies in humans and rodents; published in English language.
Result:
Finding of various studies included in our meta-analysis showed that the effect of acute, and subchronic exposure to khat showed that short-term memory appears to be affected depending on the duration of exposure. However, does not have any effect on long-term memory.
Conclusion:
Although a number of studies regarding the current topic are limited, the evidenced showed that khat (C. edulis) induced memory discrepancy.
Keywords
Catha edulis
khat
long-term memory
Qat
reference memory
short-term memory
working memory
Introduction
Khat (Catha edulis) is a psychostimulant plant grows in countries bordering the Red Sea, along the East coast of Africa, and in West Asia. It has been chewed since ancient times in Ethiopia, and its use spread to the East African countries[1] and down to South Africa.[2] It also has been used in Yemen (South of the Arabian Peninsula) since the 6th century.[3] However, recently, its use has spread to various European,[4567] Asian,[8] and Australian countries,[910] as well as to the United States.[11] The use of khat in these countries usually begins in African immigrant communities and then spreads to other residents.[12] Khat chewing is a slow-growing problem in the world, with its use growing, most rapidly in Africa.[13]
The main active substance in khat leaves is cathinone, which has structural similarity to amphetamine and is mainly responsible for most of the psychoactive properties of khat.[14] Similar to amphetamine, cathinone acts by releasing catecholamines from presynaptic storage sites, inhibiting their reuptake, thereby increasing their temporal and spatial presence at the presynaptic receptors.[15] The cathinone has also been associated directly or indirectly with dopamine or serotonin release through action on their transporter functions.[16] The neurotransmitter systems through which cathinone (khat) modulates its mechanistic processes appear to be important pathways for neurobehavioral activities including learning and memory.[17]
Users of khat report feeling as if they are able to think more clearly and more quickly while chewing khat. Moreover, khat users also reported that the acute effects of the khat include increased levels of alertness, enhanced ability to concentrate, friendliness, contentment, and flow of ideas. This is usually followed by excessive tension, anxiety, emotional instability, irritability, and restlessness within 2 h, followed by feelings of low mood, numbness, lack of concentration, sluggishness, and insomnia.[1819] Studies also reported that the use of khat has been implicated with acute, as well as chronic physio-neuro-psychological and mental outcomes.[20] The khat has been used to prevent fatigue, improve concentration and flow of ideas when studying[2122] and perceived believe in improving memory.[2324] However, some studies reported that chronic khat use has been implicated with impaired working memory and mental impairments.[1725] Although evidence from both human and animal studies has demonstrated acute and chronic khat's neurobehavioral effects, there is limited information on its effect on reversal learning and memory. We have therefore conducted this meta-analysis to determine the level of evidence for the effect of khat (C. edulis Forsk) on memory discrepancy.
Methods
Data sources and search strategy
Our systematic review and meta-analysis study was conducted according to the Cochrane guidelines[26] and has been presented based on the preferred reporting items for systematic reviews and meta-analyses checklist.[27] MEDLINE, Cochrane Library, PubMed, Academic Search Complete, SPORTDiscus, ScienceDirect, Scopus, Web of Science, and Google Scholar were searched to retrieve the papers for this review. Keywords utilized across database search were khat, cat, chat, long-term memory, short-term memory, memory deficit, randomized control trial, and cross-sectional survey. As subject headings varied between the databases, various combinations of these keywords were used. The search was limited to the studies in humans and rodents, published in English language. A search of bibliographies of acquired studies was also performed. The two reviewers (Birhane Alem Berihu and Gebrekidan Gebregzabher Asfeha) independently conducted the database searches. In addition, seven relevant journals [Figure 1] and reference lists of included studies and previous systematic reviews were manually searched. The database searching was performed since August 2015.
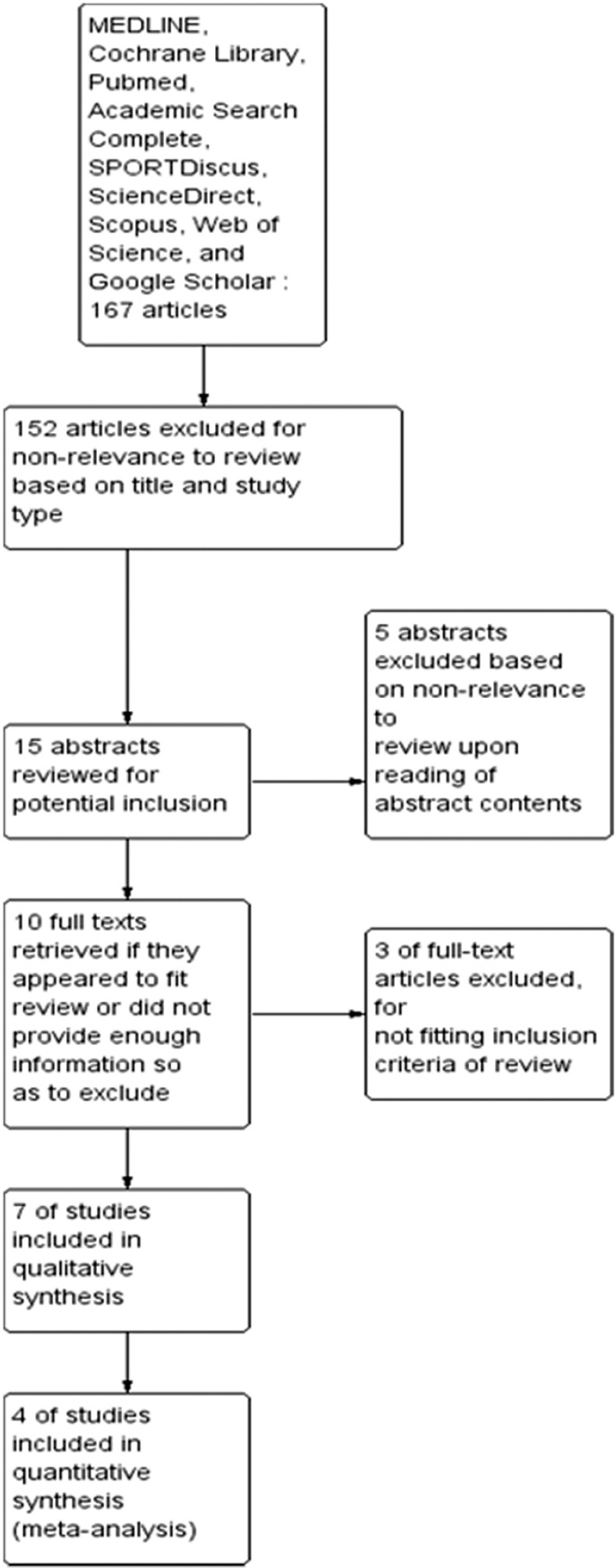
- The process of study selection according to the PRISMA flow diagram
Types of studies
To retrieve the papers for this review, studies using the randomized controlled trials (RCTs) and cross-sectional study were included in this study. Only articles published in English language limited to humans and rodent studies were included in this study. For the purpose of the present review, the focus is being about the change in long-term memory, short-term memory, and other psychological disorders. Research articles conducted on human and rodents using other drugs in addition to khat were not included. Brief trial reports, abstracts, and proceedings from the conference were excluded from the study.
Intervention
The intervention of interest was the khat effect on the short-term memory and long-term memory, such as learning memory, working memory, and other cognitive activities. Studies with khat administration did as the only intervention or with other interventions were included if the same other interventions were applied in the control group. If the exclusive effect of khat administration could not be defined in studies involving multiple interventions, those were excluded from the study.
Comparison
Control groups included no treatment or any form of intervention.
Outcome measures
Assessment of long-term memory, short-term memory or working memory, and other psychological disorders were the main outcome measures. Studies showing toxicity as at least one of outcome measures were included.
Data extraction
Two reviewers (Birhane Alem Berihu and Gebrekidan Gebregzabher Asfeha) independently reviewed all articles for eligibility [Figure 1]. For conducting meta-analysis, outcome data were assessed for eligibility, and scores were extracted from relevant included studies. One reviewer (Birhane Alem Berihu) extracted data from the included studies [Table 1] with the standardized form, and another reviewer (Gebrekidan Gebregzabher Asfeha) checked the data to ensure accuracy. Any disagreements regarding the study inclusion between two reviewers were resolved through discussion. The following were recorded from each trial where available: Intervention characteristics (khat dose, control condition, and additional treatments); participant characteristics (gender, age, and weight); study characteristics (author and publication year); outcomes (time of outcome assessment and unit of outcome assessment); and evaluation of each domain of the Cochrane risk of bias assessment tool (sequence generation, allocation concealment, blinding, incomplete outcome data, and selective outcome reporting).[28] The outcome was memory deficit, defined as reducing or increasing memory, as measured after certain days of induced memory deficit.
Data analysis
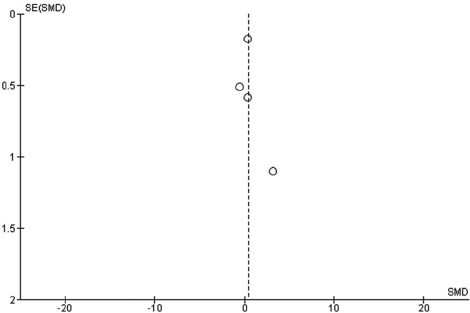
A standard mean difference (SMD) with 95% confidence interval (CI) was an effect measure used for the outcome. A weighted mean difference (WMD) with 95% CI was used to synthesize the status of memory level. The data synthesis was done with an intention to treat basis. The random effect model and a fixed effect model were used throughout the review for calculating WMDs and SMDs. The inconsistency of data was examined by looking at the graphical display of the results and also by using an I2. As recommended, an I2 of 75% or more indicate high inconsistency of data.[28] Meta-analyses were conducted to examine the effects of khat on memory in comparison with control groups. Publication bias was assessed by graphically examining the symmetry of a funnel plot. Review Manager 5.3 was used for all analyses and generating the funnel plot [Figure 2].
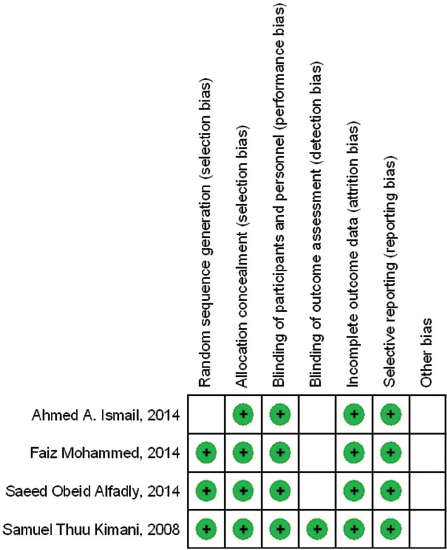
- Risk of bias summary: Review authors’ judgements about each risk of bias item for each included study
Result
Type of studies and their characteristics
The process of the selecting studies to be included in this meta-analysis and systematic review is illustrated in Figure 1. Seven articles from a total of the 167 records were included in this systematic review. The characteristics of the included studies are presented in Table 1. Five studies were RCTs, and the two studies were cross-sectional studies published in English language.
Interventions
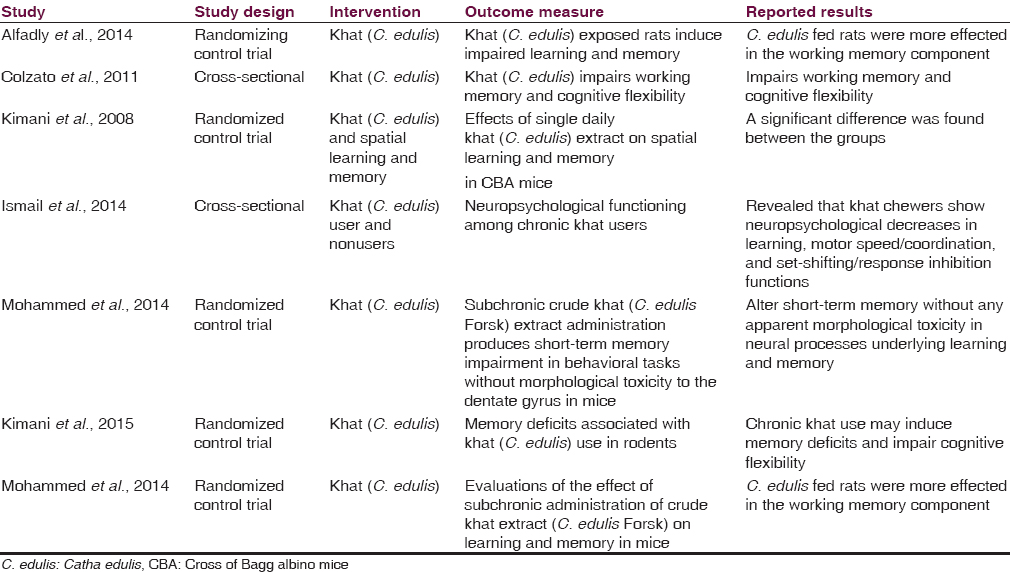
Variety in comparison interventions was shown in the present meta-analysis. These included effects of single daily khat (C. edulis) extract on spatial learning and memory in Albino mice,[29] C. edulis deteriorates spatial working memory in rats but spares reference memory,[30] khat extract administered on acute and subacute basis impaired short-term memory with no effect on long-term memory using spatial memory testing battery,[2324] neuropsychological functioning among chronic khat users in Jazan Region, Saudi Arabia,[31] memory deficits associated with khat (C. edulis) use in rodents,[32] and khat use impairs memory and flexibility.[33]
Primary outcomes
Studies analyzed in this review revealed that khat (C. edulis) shows a significant effect on short-term memory or working memory but not long-term memory.
Risk of bias item for each included study
Figure 3 shows the summary of assessing the risk of bias. As studies successfully perform selection bias, allocation concealments, blinding of participants and personnel, attrition bias, and reporting bias, there were low risk of performance bias and detection bias across the included studies.
- Funnel plot for effect of the Khat (Catha edulis) on memory deficit
Publication bias
It was difficult to determine if there was publication bias from the funnel plot because of the small numbers of studies [Figure 2].
Effect of the khat (Catha edulis) on short- and long-term memory
Of the seven included studies, four studies were suitable for meta-analysis. As heterogenicity was evidenced in all meta-analyses (heterogeneity: τ =0.49; χ2 = 9.39, df = 3 (P = 0.02); I2 =68%), [Figure 4] the random effect model was used and it generated the weights from inverse variance weighting. In terms of the effects of khat (C. edulis) on short-term memory in comparison with the control group, four studies were identified.[23293031] The overall estimate of the effect khat on short-term memory suggested that it is responsible for induction of the short-term memory deficit. Moreover, of these four studies, three studies were also identified to assess its effect on long-term memory.[233031] As homogenicity was evidenced in all meta-analyses (heterogeneity: χ2 = 0.50, df = 2 [P = 0.78]; I2 =0%) [Figure 5], the fixed effect model was used and it generated the weights from inverse variance weighting. The overall estimate of the effect on long-term memory test for overall effect: Z = 2.32 (P = 0.02) [Figure 5] suggested that there was no significant difference between khat exposed and control group for the induction of long-term memory deficit.
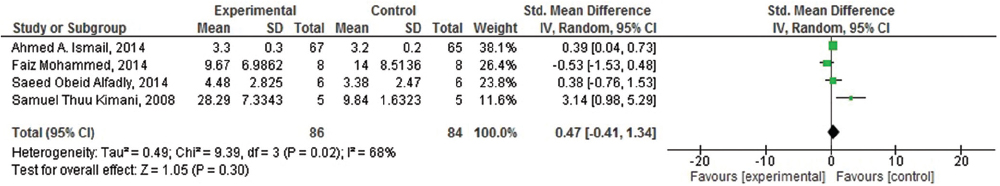
- Forest plot of comparison: Meta-analysis on effect of Khat (Catha edulis) on short term memory deficit
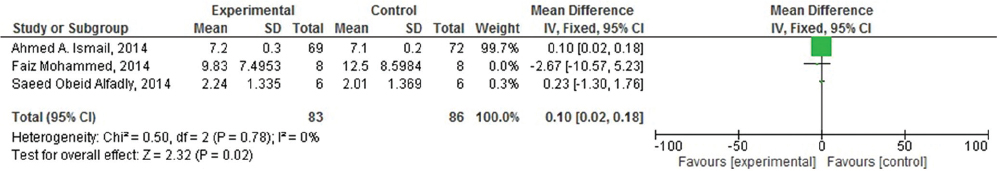
- Forest plot of comparison: Effect of Khat (Catha edulis) on long term memory deficit
Discussion
This systematic review and meta-analysis was conducted to determine the level of evidence of the effect of khat (C. edulis Forsk) on memory discrepancy. Our meta-analysis results suggest that there is evidence of the khat (C. edulis Forsk) induced short-term memory discrepancy. Conversely, the results also suggest that the khat (C. edulis Forsk) does not have any effect on long-term memory. As the number of studies included in the analyses is very small, it is reasonable to think that these somewhat conflicting results may represent a lack of research regarding the effects of khat (C. edulis Forsk) on memory discrepancy rather than demonstrating actual effects. Finding of various studies included in our meta-analysis showed that the effect of acute, subacute, and subchronic exposure to khat (C. edulis Forsk) showed that short-term memory appears to be affected depending on the duration of exposure. However, it does not have any effect on long-term memory.[232425303132]
Some limited studies and single case reports suggest long-term effects of khat on behavioral and cognitive functions. These effects range from minor to major psychiatric problems. Minor effects include insomnia, anxiety, irritability, agitation, and aggression. Other documented psychiatric effects include a short-lived schizophreniform psychotic illness and mania.[3334] The use of an excessive amount of khat has also been found to significantly increase the risk of psychosis or psychotic-like episodes,[35] including paranoid delusions and significant mania. These effects are similar to those observed in amphetamine users. Depression has also been frequently reported to be related to long-term use of khat and seems to be particularly evident during cessation.[36]
A comparative analysis of spatial acquisition, reversal learning, and reference (long-term) memory in khat (C. edulis Forsk) treated versus control mice is reported. The results showed that reference memory during postacquisition phase was retained across the treatment regimes. However, khat (C. edulis Forsk) extract inhibited both learning and reference memory during the reversal phase in a dose-dependent manner. These differential effects of the extract on spatial learning and memory may be attributed to short, as well as long-term deleterious effects of khat on working memory and cognitive flexibility.[1725] A recent systematic review and meta-analysis of the neurobehavioral deficits associated with exposure to khat (C. edulis Forsk) showed that the studies conducted on rodents and human implicate a significant effect of khat use on cognitive flexibility, working memory, learning memory, motor activities, and other psychological disorders.[37] Recent findings have also shown that khat extract administered on acute and subacute basis impaired short-term memory with no effect on long-term memory using spatial memory testing battery.[2324] Bearing in mind the similarity in pharmacological components of khat (cathinone and cathine) and amphetamines, the findings analyzed in our meta-analysis are consistent with previous studies in humans showing impairments in working memory[383940] and cognitive flexibility[41] as consequences of long-term amphetamine and methamphetamine use. Comparable studies of systematic review and meta-analysis of the neurocognitive deficits associated with users of methamphetamines indicate that the largest (medium to large) effect sizes are seen in the domains of executive functions, learning, and memory.[42] The extract may have also modulated its effect, possibly through structural modification of the brain similar to amphetamines[43] and/or khat (C. edulis Forsk) induced mental impairment.[2144]
A number of studies have suggested that chronic methamphetamine use is associated with mild to moderate neuropsychological impairment, with one current estimate suggesting that approximately 40% of persons with methamphetamine dependence demonstrate some level of global neuropsychological impairment.[45] Significant deficits in several different cognitive processes dependent on brain frontostriatal and limbic circuits have been observed in studies of chronic methamphetamine users, including deficits in psychomotor functions, complex information processing speed, attention and working memory, episodic memory, and executive functions, including response inhibition and novel problem-solving.[464748] Neural-behavioral changes seen in amphetamine users have been attributed to altered dopaminergic transmission in the prefrontal cortex and/or striatum[49] in the absence of reduced motivation and impaired cognitive performance.[50] Such neurochemical changes may represent neuro-adaptive changes developed during repeated psychostimulants exposure. The brain structures known to undergo adaptive changes during repeated amphetamine administration, including the striatal complex, prefrontal cortices, and limbic areas, have also been implicated in various forms of learning and memory.[5152] Repeated psychostimulants use and/or abuse, including amphetamine and possibly amphetamine-like khat, have been implicated with psychopathology resembling acute schizophrenic phenotype resulting from structural, molecular, neurochemical, and neurophysiological changes.[53] Cognitive impairment is a key feature of patients with schizophrenia and an important determinant of the outcome and a target for therapy.[54]
The impairment is attributable to diffuse abnormalities in frontal cortex, hippocampus, and other cognitive modulating structures.[55] Physiologically, the impairments are associated with deficits in glutamatergic, GABAergic, dopaminergic, cholinergic, and serotonergic neurotransmitter systems. The serotonergic neurotransmitter system contributes to the deficit through its influence on dopaminergic, cholinergic, glutamatergic, GABAergic, and growth factors and receptor functions all important in schizophrenia and cognitive status.[2256] Additional studies are required to determine the specific neural mechanisms responsible for the observed patterns of learning and memory. The different patterns of memory deficits may reflect the differences impact of dose effect, as well as a time-dependent impairment. Further studies are needed to whether these findings included in our meta-analysis are as a result of this mechanism remains to be elucidated.
Conclusion
Finding of various studies included in our meta-analysis showed that the effect of acute and subchronic exposure to khat showed that short-term memory appears to be affected depending on duration of exposure. However, it shows no effect on long-term memory. Although a number of studies regarding the current topic are limited, the evidenced showed that khat (C. edulis) induced memory discrepancy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors/editors/publishers of all those articles, journals, and books from where the literature for this article has been reviewed and discussed.
References
- Khat in the Horn of Africa: historical perspectives and current trends. J Ethnopharmacol. 2010;132:607-14.
- [Google Scholar]
- The use of khat (Catha edulis) in Yemen. Social and medical observations. Ann Intern Med. 1976;85:246-9.
- [Google Scholar]
- Khat habit and its health effect. A natural amphetamine. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2004;148:11-5.
- [Google Scholar]
- Khat use and monitoring drug use in Europe: the current situation and issues for the future. J Ethnopharmacol. 2010;132:578-83.
- [Google Scholar]
- Risk assessment of khat use in the Netherlands: a review based on adverse health effects, prevalence, criminal involvement and public order. Regul Toxicol Pharmacol. 2008;52:199-207.
- [Google Scholar]
- Khat in the neighbourhood – Local government responses to khat use in a London community. Subst Use Misuse. 2008;43:819-31.
- [Google Scholar]
- Khat chewing: an emerging drug concern in Australia? Aust N Z J Psychiatry. 2005;39:842-3.
- [Google Scholar]
- Psychoses, PTSD, and depression in Somali refugees in Minnesota. Soc Psychiatry Psychiatr Epidemiol. 2011;46:481-93.
- [Google Scholar]
- Perceptions of the use of khat among Somali immigrants living in Swedish society. Scand J Public Health. 2011;39:212-9.
- [Google Scholar]
- United Nations Office on Drugs and Crime (UNODC). World Drug Report. Vienna: UNODC; 2011.
- A survey of medication treatment for hyperactive/inattentive students. JAMA. 1988;260:2256-8.
- [Google Scholar]
- Anticataleptic activity of cathinone and MDMA (Ecstasy) upon acute and subchronic administration in rat. Synapse. 2003;49:232-8.
- [Google Scholar]
- Working memory and speed of information processing in chronic khat users: preliminary findings. Eur Addict Res. 2013;19:1-6.
- [Google Scholar]
- Catha edulis, an international socio-medical problem with considerable pharmacological implications. East Afr Med J. 1991;68:555-61.
- [Google Scholar]
- Amphetamine-like effects in humans of the khat alkaloid cathinone. Br J Clin Pharmacol. 1990;30:825-8.
- [Google Scholar]
- Molecular targets for treating cognitive dysfunction in schizophrenia. Schizophr Bull. 2007;33:1100-19.
- [Google Scholar]
- Subchronic crude khat (Catha edulis f.) extract administration produces short-term memory impairment in behavioral tasks without morphological toxicity to the dentate gyrus in mice. Ethiop Pharm J. 2014;30:77-94.
- [Google Scholar]
- Evaluations of the effect of subchronic administration of crude khat extract (Catha edulis f.) on learning and memory in mice. Neurology. 2015;84(14 Suppl):077.
- [Google Scholar]
- Khat use is associated with impaired working memory and cognitive flexibility. PLoS One. 2011;6:e20602.
- [Google Scholar]
- Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011. Available from http://handbook.cochrane.org
- Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
- [Google Scholar]
- Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Chichester, UK: John Wiley & Sons, Ltd; 2011. The Cochrane Collaboration
- [Google Scholar]
- Effects of single daily khat (Catha edulis) extract on spatial learning and memory in CBA mice. Behav Brain Res. 2008;195:192-7.
- [Google Scholar]
- Catha edulis deteriorates spatial working memory in rats, but spares reference memory. Indian J Physiol Pharmacol. 2014;58:240-50.
- [Google Scholar]
- Neuropsychological functioning among chronic khat users in Jazan region, Saudi Arabia. Subst Abus. 2014;35:235-44.
- [Google Scholar]
- Memory deficits associated with khat (Catha edulis) use in rodents. Metab Brain Dis. 2016;31:45-52.
- [Google Scholar]
- Chronic khat use and psychotic disorders: A review of the literature and future prospects. Sucht. 2007;53:9-22.
- [Google Scholar]
- The consumption of khat and other drugs in Somali combatants: a cross-sectional study. PLoS Med. 2007;4:e341.
- [Google Scholar]
- Khat use as risk factor for psychotic disorders: a cross-sectional and case-control study in Somalia. BMC Med. 2005;3:5.
- [Google Scholar]
- Effect of khat (Catha edulis Forsk) on neurobehavioral functions: Systematic review and meta-analysis. Int J Pharma Sci Res. 2015;6:1369-77.
- [Google Scholar]
- Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: a review. J Neuropsychiatry Clin Neurosci. 2003;15:317-25.
- [Google Scholar]
- Neural mechanisms of working memory in ecstasy (MDMA) users who continue or discontinue ecstasy and amphetamine use: evidence from an 18-month longitudinal functional magnetic resonance imaging study. Biol Psychiatry. 2004;56:349-55.
- [Google Scholar]
- Neurochemical similarities between d, l-cathinone and d-amphetamine. Drug Alcohol Depend. 1982;9:279-84.
- [Google Scholar]
- Executive control deficits in substance-dependent individuals: a comparison of alcohol, cocaine, and methamphetamine and of men and women. J Clin Exp Neuropsychol. 2009;31:706-19.
- [Google Scholar]
- Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17:275-97.
- [Google Scholar]
- A combined spectroscopic and functional MRI investigation of the dorsal anterior cingulate region in opiate addiction. Mol Psychiatry. 2007;12:611, 691-702.
- [Google Scholar]
- Use of khat and posttraumatic stress disorder as risk factors for psychotic symptoms: a study of Somali combatants. Soc Sci Med. 2009;69:1040-8.
- [Google Scholar]
- Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc. 2004;10:1-14.
- [Google Scholar]
- Cognitive deficits among methamphetamine users with attention deficit hyperactivity disorder symptomatology. J Addict Dis. 2002;21:75-89.
- [Google Scholar]
- Cognitive impairment in individuals currently using methamphetamine. Am J Addict. 2000;9:222-31.
- [Google Scholar]
- Deficient strategic control of verbal encoding and retrieval in individuals with methamphetamine dependence. Neuropsychology. 2005;19:35-43.
- [Google Scholar]
- Increased conditioned fear response and altered balance of dopamine in the shell and core of the nucleus accumbens during amphetamine withdrawal. Neuropharmacology. 2002;42:633-43.
- [Google Scholar]
- Long-term potentiation alters the modulator pharmacology of AMPA-type glutamate receptors. J Neurophysiol. 2002;87:2790-800.
- [Google Scholar]
- Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997;17:8491-7.
- [Google Scholar]
- Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515-32.
- [Google Scholar]
- An escalating dose “binge” model of amphetamine psychosis: behavioral and neurochemical characteristics. J Neurosci. 1997;17:2551-66.
- [Google Scholar]
- Does stimulation of 5-HT(1A) receptors improve cognition in schizophrenia? Behav Brain Res. 2008;195:98-102.
- [Google Scholar]
- Effects of serotonin depletion on the hippocampal GR/MR and BDNF expression during the stress adaptation. Behav Brain Res. 2008;195:129-38.
- [Google Scholar]






