Translate this page into:
Assessment of intracranial metastases from neuroendocrine tumors/carcinoma
This is an open access article distributed under the terms of the Creative Commons Attribution NonCommercial ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
The most common sites of origin for neuroendocrine carcinoma are gastrointestinal tract and its accessory glands, and lungs.
Materials and Methods:
One-hundred fifty cases diagnosed with metastatic brain lesions were retrieved from hospital records within 5 years. For these cases, the primary neoplasm, histopathological classification, metastasis, treatment, and fate all were studied.
Results:
Intracranial deposits were detected in 10%. The primary lesion was in the lungs in 87% of patients, and 1 patient in the breast and 1 in esophagus. Pathological classification of the primary lesion was Grade 2 (MIB-1: 3–20%) in 1 patient and neuroendocrine carcinoma (MIB-1: ≥21%) in 14 patients. The median period from onset of the primary lesion up to diagnosis of brain metastasis was 12.8 months. About 33% of patients had a single metastasis whereas 67% patients had multiple metastases. Brain metastasis was extirpated in 33% of patients. Stereotactic radiotherapy alone was administered in 20% of patients, and brain metastasis was favorably controlled in most of the patients with coadministration of cranial irradiation as appropriate. The median survival period from diagnosis of brain metastasis was 8.1 months.
Conclusion:
Most of patients with brain metastasis from neuroendocrine carcinoma showed the primary lesion in the lungs, and they had multiple metastases to the liver, lymph nodes, bones, and so forth at the time of diagnosis of brain metastasis. The guidelines for accurate diagnosis and treatment of neuroendocrine carcinoma should be immediately established based on further analyses of those patients with brain metastasis.
Keywords
Intracranial metastases
neuroendocrine carcinoma
radiotherapy
Introduction
Neuroendocrine tumor (NET)/carcinoma is characterized by secretion of many active hormones and the presence of intracytoplasmic secretory granules.[1]
They may develop in all body organs, and many of them also have a malignant course. The World Health Organization (WHO) therefore attempted to grade NETs based on systematic clinical pathological classification, but classifications with different criteria were proposed for NETs originating from the favored sites (pancreas and gastrointestinal tract) and NETs originating from the lungs.
Neurosurgeons are increasingly experiencing patients with brain metastasis from tumors diagnosed as neuroendocrine carcinoma.[234567] However, few reports examined brain metastasis of such patients.
Materials and Methods
All cases of neuroendocrine carcinoma were searched among histopathological diagnoses of all neoplasm specimens obtained during surgical procedures at Kameda Hospital and Oncology Center; Kamogawa – JAPAN during the 5 years between January 2011 and December 2015. As a result, 150 patients were confirmed carrying their diagnosis. The age of patients ranged between 21 and 91 years (median: 64.5-year-old) and there were 90 men and 60 women. The primary site of NETs was the lungs in 51 patients, rectum in 18 patients, pancreas in 13 patients, stomach in 10 patients, mammary glands in 10 patients, uterus in 9 patients, lymph nodes in 8 patients, and duodenum in 6 patients. Other sites of onset were the thymus gland in 4 patients, esophagus in 3 patients, ileum in 3 patients, liver in 2 patients, colon in 2 patients, pharynx in 2 patients, paranasal sinus in 1 patients, kidneys in 1 patients, urinary tract in 1 patients, and ovaries in 1 patients and 5 cases of unknown primary.
This study was conducted in Kameda Hospital and Oncology Center; Kamogawa-Chiba-JAPAN.
Results
Clinical data
Age of patients ranged between 34 and 78 years (median: 68-year-old) and 20 of them were men and 10 of them were women [Table 1].
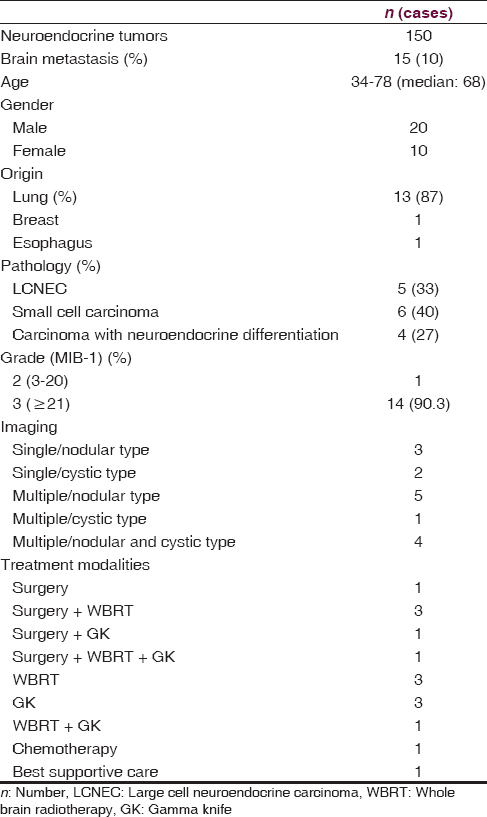
The period from histopathological detection of primary lesion up to diagnosis of brain metastasis ranged between 0 and 33.4 months (median: 12.8 months) [Figure 1].

- Period from histopathological diagnosis of the primary tumor till discovery of intracranial deposits
Fate and outcomes
The follow-up period after treatment of metastatic brain tumors was from 1 to 34 months (median: 5.8 months), and 29 of 31 patients were deceased by the time of completion of examinations. Analysis by the Kaplan–Meier survival curve revealed a median survival period of 8.1 months [Figures 2–5].

- Radiologic patterns. (a) Single/nodular type. (b) Single/cystic type. (c) Multiple/nodular type. (d) Multiple/cystic type

- Survival period after diagnosis of metastatic brain tumor

- (a) Chest computed tomography scan shows a small lesion on the mediastinum at right S2. (b) Sagittal contrast-enhanced T1-weighted head magnetic resonance imaging reveals two tumors in the pineal body and the cerebellum accompanied by obstructive hydrocephalus. (c) H and E, stain image of neuroendoscopic biopsy tissues (—200). (d) anti-synaptophysin antibody stain image (—200). (e) Anti-chromogranin A antibody stain image (—200). (f) MIB-1 stain image (×200). (g) Bone scintigraphy shows multiple metastases to the cervical vertebrae

- (a and b) Horizontal contrast-enhanced T1-weighted head magnetic resonance imaging shows multiple tumors with cysts in the right occipital lobe and the parietal lobe. (c) H and E, image of the isolated parietal lobe tumor tissues (—200). (d) Anti-synaptophysin antibody stain image (—200). (e) Anti-CD56 antibody stain image (—200). (f) Anti-TTF-1 antibody stain image (—200). (g and h) Horizontal contrast-enhanced T1-weighted head magnetic resonance imaging after cranial irradiation therapy
Discussion
Carcinoid has been the description of NETs for any years. It was later considered that NETs should be treated as clinically malignant tumors and the term “carcinoid” was eliminated from the WHO classification in 2000. Subsequently, NETs were categorized into five types, namely, NET Grade 1, NET Grade 2, NEC (large cell or small cell), mixed adenoneuroendocrine carcinoma, and hyperplastic and neoplastic lesions, in the 2010 WHO classification according to the grading by the Ki-67 labeling index.[8]
The incidence of NETs among all tumors was 4.44% in Caucasians and 6.5% in African Americans, and male patients were slightly predominant; the incidence increased by 37% in Caucasians and 40% in African Americans compared with the statistical data between 1993 and 1997.[9] The site of onset of NETs in Caucasian patients was the lungs/bronchus in 31.9%, small intestine in 17.7%, unknown in 13.5%, and rectum in 12.3% whereas that in African American patients was the rectum in 27%, small intestine in 21%, and lungs/bronchus in 18.3%. Thus, a slight ethnic difference was observed.[9] Also, in the percentage of NETs against all types of tumors in each site of origin, NETs accounted for the highest (1.42%) in the lungs/bronchus in Caucasian patients whereas NETs accounted for the highest (1.65%) in the rectum in African American patients.[9] The pancreas accounted for 4% of all types of the NETs and for approximately 0.3% of all pancreatic cancers,[9] being a very rare disease. Regarding the metastasis of NETs to other organs, distant metastasis was observed in 20–30% of patients at the time of diagnosis.
We retrieved 150 patients with NETs who were diagnosed at our hospital over the past 5 years. The site of onset was the lungs in 51 patients, rectum in 18 patients, and pancreas in 13 patients, which were almost comparable with the site of onset in Caucasian patients, and the number of patients with NETs in the pancreas was higher in Japanese patients. Brain metastasis was observed in 15 patients (10.0%) and it was interesting that 13 (87%) of them were metastasis from the primary lung NETs. Primary brain metastasis is mainly caused by primary lung NETs.
The European Neuroendocrine Tumor Society (ENETS) in 2012 published the consensus guidelines for the diagnosis and treatment of NETs for each site of onset.[10] During preparation of the guidelines, the ENETS advisory board, and experts have published the analytical results on metastasis to other organs from NETs.[1011] The description of brain, and ovarian metastasis in the article stated that brain metastasis from primary lung NETs accounted for 45–71% of patients with the median period of 1.5 years from the diagnosis of the primary lesion until confirmation of brain metastasis.[8] Lymph node metastasis and liver metastasis were present in 75% and 50% of the patients with brain metastasis,[11] respectively, which were comparable to the results of our present study. Approximately 1.4% of metastatic brain tumors were NETs whereas the incidence of brain metastasis from NETs was 1.5–5%.[11] The incidence of primary lung NETs was higher in our present study and that would be the cause of slightly higher incidence in brain metastasis at 10.3%. A report that summarized 24 patients was the highest number of patients and only 57 patients were reported during the 46 years between 1962 and 2007.[11] The report of ENET[11] did not contain a definite description of pathological findings and grading.
When limited to lung NETs, brain metastasis occurs in 30% or more of the large cell neuroendocrine carcinoma (LCNEC) patients and in 24% of the small cell carcinoma patients.[312] In the present study, the strategy of surgery and radiotherapy for the patients was determined based on the symptoms, PS, and imaging findings (number of brain metastases), and such action would be the standard concept for metastatic brain tumors at present. On the other hand, prophylactic cranial irradiation is also a possible option for small cell lung cancer.[3121314] The median survival period from diagnosis of brain metastasis was 8.1 months in the present study, and it was not greatly different from that of brain metastasis in all lung cancer patients.[13] Lately, various therapies such as combination chemotherapy, epidermal growth factor receptor-tyrosine kinase inhibitors, and angiogenesis inhibitors have become available for brain metastasis originating from lung cancer.[131415]
Conclusion
Approximately 10% of NET patients developed brain metastasis at slightly longer than 1 year after diagnosis, and metastasis originating from LCNEC or small cell carcinoma occurred in most of them. The treatment options for brain metastasis varied depending on the symptoms due to brain metastasis, performance status, and metastatic images. Most of the metastases were controlled by applying active therapy to the brain lesion, but the mean survival period was 8 months from diagnosis of brain metastasis, mainly because of aggravation of the primary lesion or metastasis to other organs. Standardization of clinical and pathological interpretation of NETs can give better help by establishment of optimal guidelines for diagnosis and treatment of brain metastasis originating from NETs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Brain carcinoid metastases: Outcomes and prognostic factors. J Neurosurg. 2013;118:889-95.
- [Google Scholar]
- Chemotherapy for pulmonary large cell neuroendocrine carcinoma: Similar to that for small cell lung cancer or non-small cell lung cancer? Lung Cancer. 2012;77:365-70.
- [Google Scholar]
- Pulmonary large cell neuroendocrine carcinoma diagnosed in a brain metastasis. Int J Clin Exp Pathol. 2012;5:159-62.
- [Google Scholar]
- Brain metastasis from large cell neuroendocrine carcinoma of the urinary bladder. Surg Neurol Int. 2011;2:84.
- [Google Scholar]
- Typical bronchial carcinoid metastasizing to the brain: A case presentation. Case Rep Oncol. 2011;4:602-10.
- [Google Scholar]
- NANETS consensus guidelines for the diagnosis of neuroendocrine tumor. Pancreas. 2010;39:713-34.
- [Google Scholar]
- Neuroendocrine tumor epidemiology: Contrasting Norway and North America. Cancer. 2008;113:2655-64.
- [Google Scholar]
- European Neuroendocrine Tumor Society. Available from: http://www.enets.org
- ENETS consensus guidelines for the management of brain, cardiac and ovarian metastases from neuroendocrine tumors. Neuroendocrinology. 2010;91:326-32.
- [Google Scholar]
- Biological characteristics and treatment outcomes of metastatic or recurrent neuroendocrine tumors: Tumor grade and metastatic site are important for treatment strategy. BMC Cancer. 2010;10:448.
- [Google Scholar]
- Clinicopathological analysis in patients with neuroendocrine tumors that metastasized to the brain. BMC Cancer. 2016;16:36.
- [Google Scholar]
- Bone and brain metastasis in lung cancer: Recent advances in therapeutic strategies. Ther Adv Med Oncol. 2014;6:101-14.
- [Google Scholar]






