Translate this page into:
Tetraventricular central neurocytoma: A rare presentation with imaging-pathologic correlation
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Central neurocytoma (CN) is a benign intraventricular neuronal tumor with a favorable prognosis. It accounts approximately 0.25–0.5% of intracranial tumors. In this report, we describe a very rare case of tetraventricular CN with imaging-pathologic correlation, and discuss their atypical features in a location together with treatment options. A 27-year-old man was admitted to the hospital with symptoms of progressive headaches of several months’ duration. Magnetic resonance imaging of the brain revealed a well-circumscribed, lobulated intraventricular mass with numerous intratumoral cystlike areas. The mass was located in the enlarged lateral ventricles bilaterally extending to the third and the fourth ventricle. Surgical removal of the 4th ventricle component of the tumor was performed. Histomorphological and immunohistochemical findings of the tumor were consistent with CN. After pathological diagnosis, gamma knife surgery was performed. CN may present with atypical features in a location with a usual histopathological findings. To our knowledge, we described the third case of tetraventricular CN, which was partially treated with both surgical resection and radiosurgery.
Keywords
Neurocytoma
radiosurgery
surgery
ventricle
Introduction
Central neurocytoma (CN) is a benign intraventricular neuronal tumor with a favorable prognosis. They are typically seen in young adults and the median age at diagnosis is 34 years with a wide range. It accounts approximately 0.25–0.5% of intracranial tumors.[1] CNs are designated as World Health Organization (WHO) Grade II neoplasm, and their preferential localization is the lateral ventricle body.
In this report, we describe a very rare case of tetraventricular CN with imaging-pathologic correlation, and discuss their atypical features in a location together with treatment options.
Case Report
A 27-year-old man was admitted to the hospital with symptoms of progressive headaches of several months’ duration. The patient reported a history of cranial operation following gamma knife surgery for an intraventricular tumor without histopathological diagnosis 4 years prior to the current admission. Magnetic resonance imaging (MRI) of the brain revealed a well-circumscribed, lobulated intraventricular mass with numerous intratumoral cystlike areas. The mass was located in the enlarged lateral ventricles bilaterally extending to the third and the fourth ventricle. The lesion was mixed inhomogeneous signal intensity on T2-weighted and fluid-attenuated inversion recovery MRIs [Figure 1], and unenhanced T1-weighted MRIs [Figure 2a–c]. There was a mild enhancement on gadolinium-enhanced T1-weighted MRIs [Figure 2d–f]. MRI also revealed partially restricted diffusion that was heterogeneous hyperintense to brain on diffusion-weighted imaging (DWI) and hypointense on corresponding apparent diffusion coefficient (ADC) maps [Figure 3a and b]. Noncontrast computed tomography (CT) scan demonstrated diffuse and diverse calcification of the lesion [Figure 3c]. Preoperative imaging of the other neuraxis was unremarkable.
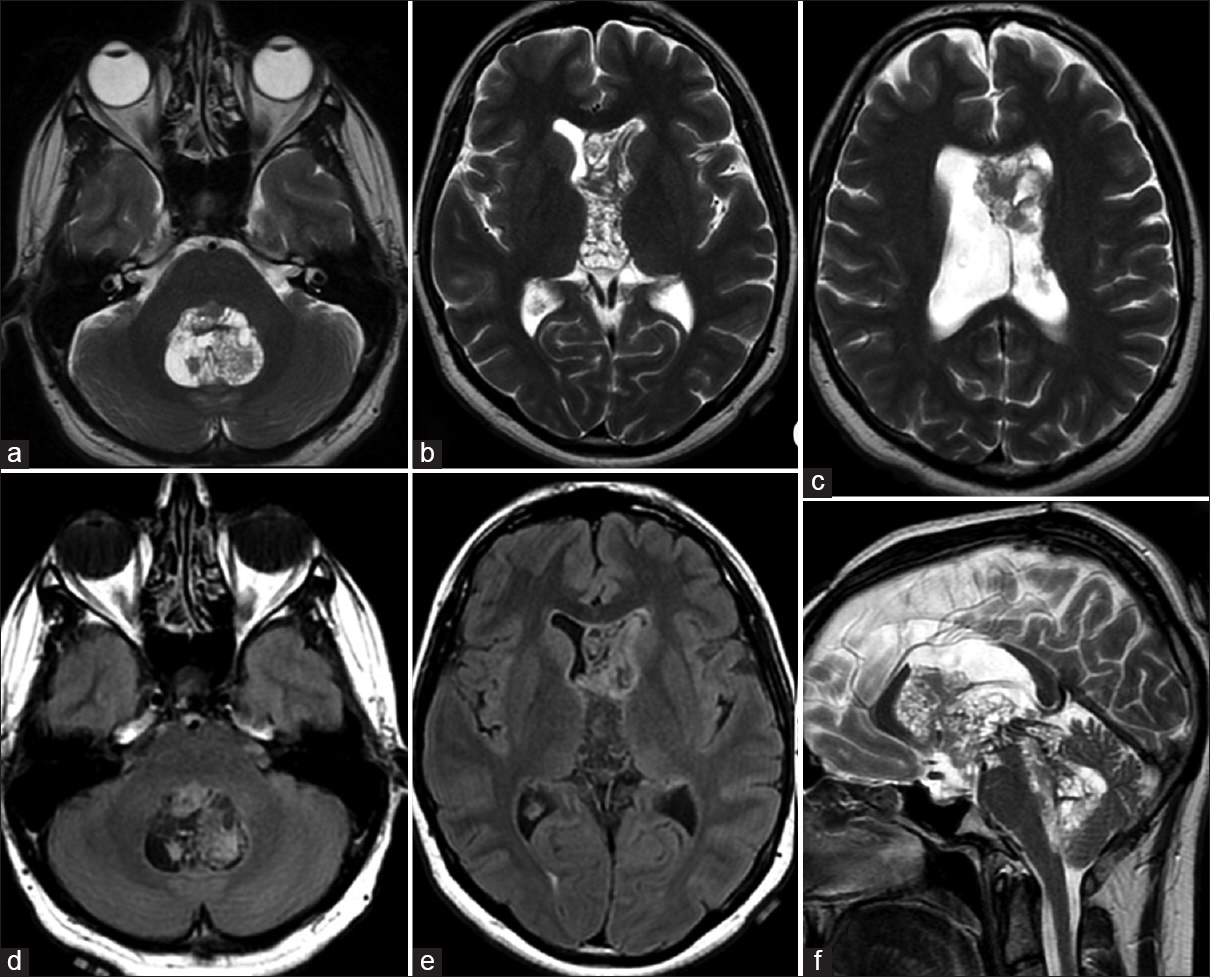
- Transverse T2-weighted (a-c) and fluid-attenuated inversion recovery (d and e) magnetic resonance images demonstrate well-circumscribed, lobulated tetraventricular mass with inhomogeneous signal intensities. Tetravenricular central neurocytoma is observed on sagittal T2-weighted magnetic resonance (f). There is “bubbly” appearance due to numerous intratumoral cystlike areas

- Transverse unenhanced (a-c) and contrast enhanced (d-f) T1-weighted magnetic resonance images demonstrate well-circumscribed, lobulated tetraventricular mass with inhomogeneous signal intensities. There is mild enhancement on contrast-enhanced T1-weighted magnetic resonance images

- The tumor is heterogeneous hyperintense to brain on diffusion-weighted imaging (a) and hypointense on corresponding apparent diffusion coefficient map (b) consistent with partially restricted diffusion. Transverse computed tomography scan demonstrates (c) diffuse and diverse calcification of the lesion
On the basis of these imaging findings, the patient underwent reoperation via suboccipital craniectomy. Surgical removal of the 4th ventricle component of the tumor was performed.
Histopathological examination revealed a neuroepithelial tumor composed of uniform small, round cells in a delicate fibrillary matrix with extensive calcification [Figure 4a]. Capillary-sized blood vessels were seen in a linear arborizing pattern throughout the tumor. Perivascular nucleus-free areas were found in some part of the tumor. Most of the tumor cells showed oligodendroglioma-like features with honey-comb appearance [Figure 4b]. Rare mitotic figures were found in the tumor, but necrosis or vascular endothelial proliferation was not observed. Immunohistochemical staining results showed diffuse positivity of the tumor cells and their processes for synaptophysin [Figure 4c]. No immunoreactivity was seen in the tumor cells for chromogranin A for neurofilament protein or glial fibrillary acidic protein (GFAP). Reactive astrocytes were identified by GFAP-immunoreactive cell processes in the perivascular location. The proliferation index (Ki67) was 4% [Figure 4d]. Histomorphological and immunohistochemical findings of the tumor were consistent with CN.

- Photomicrographs of the biopsy specimen. (a) H and E, ×100. Significant calcification within the tumor composed of small and round darkly stained nuclei. (b) H and E, ×400. Round to oval tumor cells having central nuclei with a moderate amount of clear cytoplasm. (c) Synaptophysin, ×200. Immunoreactivity for synaptophysin of tumor cells. (d) Ki67, ×400. Ki67 immunopositive tumor cells
Discussion
Intraventricular tumors may arise from a variety of cells in the region. Most of them are of nonneuronal origin such as oligodendroglioma, ependymoma, meningioma, choroid plexus papilloma, and giant cell astrocytoma. However, intraventricular primary neuronal tumors are considered to be rare. CNs were first defined by Hassoun et al. in 1982. They described two cases of primary calcified intraventricular tumors with features of neuronal differentiation.[2] The term CN is reserved for neurocytomas that occur in the ventricular system. Similar neoplasms may locate outside the ventricular system, and the WHO 2007 designated the term “extravenricular neurocytoma” for them as a variant of CN.
Although neurocytoma cells most commonly originate from remnants of the subependymal plate or from nuclei of gray matter in the septum pellucidum and lateral ventricle, the exact origin of these tumors remains unclear. The results of cell-culture investigations suggest they arise from bipotential progenitor cells that are capable of both neuronal and glial differentiation.[3]
CNs typically occur in the lateral ventricle with or without extension into the third ventricle.[4] Approximately fifty percent of CNs is located in the anterior portion of a lateral ventricle around foramen of Monro, 15% in both lateral and 3rd ventricles, and 15% are bilateral.[5] Rare locations of CNs have also been reported including the temporal horn of lateral ventricle, 3rd ventricle in isolation, 4th ventricle in isolation, and 3rd and 4th ventricles with aqueduct involvement.[6789] Tetraventricular involvement of the disease is extremely rare and to our knowledge only two cases were reported in the literature.[1011] This is the third tetraventricular CN case with classical neuroimaging and histopathological findings including DWI. On DWI sequence, the tumor shows heterogeneous high signal intensity to the brain with a corresponding low ADC value consisted with restricted diffusion.[12]
On MRI, CN appears as a heterogeneous well-circumscribed, lobulated mass isointense or slightly hypointense to gray matter on T1-weighted images and hyperintense on T2-weighted images. Patients with larger tumors may have a “bubbly” appearance due to the presence of multiple cysts like areas. On CT, these tumors are usually hyperattenuating compared to white matter. Calcification is seen in over half of cases, usually diffuse and diverse in nature and readily detected on CT scan. Although varying degrees of contrast material enhancement in CN have been reported, moderate to strong heterogeneous enhancement is usually seen on imaging studies. Prominent flow voids, increased T2 signal intensity in the adjacent periventricular white matter and intratumoral hemorrhage may also be seen. Hydrocephalus is a common finding.[15]
Recent MR spectroscopy studies have revealed the presence of glycine and alanine with increased choline, glutamate, and glutamine complex in CNs, which is probably a reflection of brain damage or a disturbance of neurotransmitting mechanisms. The classic neuro-imaging findings and the presence of a characteristic peak in the spectrum at 3.55 ppm from an elevation of glycine associated with appropriate clinical setting may differentiate CNs from other intraventricular neoplasms.[1512]
Although multi-modality treatments have been offered for CNs, surgery is the primary option for the treatment. The main aims of surgery are to establish the CSF pathway, determine the histological diagnosis and accomplish maximal surgical resection with minimum risk of neurological impairment. A transcortical-transventricular and transcallosal-transventricular routes are the most common surgical approaches for unilaterally located tumors in the anterior part of lateral ventricles. An interhemispheric-transcallosal-transventricular approach may become necessary for the bilateral tumors or in the cases which extend to the third ventricle. In our case, as the complete resection of the tumor seemed to be impossible, we preferred to operate the 4th ventricle component of the tumor to confirm the histopathological diagnosis with minimum risk of neurological impairment.
Fractionated radiotherapy and stereotactic radiosurgery can be considered for residual tumors, especially those with anaplastic features.[413] However, we avoided using the fractionated radiotherapy due to its risks in a benign tumor in the presented young man since the radiotherapy field was too large. Instead of the radiotherapy choice, gamma knife surgery was preferred, since the tumor control rate following gamma knife surgery is high, and the risk-benefit relationship compares favorably to that following radiotherapy of CN, even when low prescribed doses are used.[14] The patient is still in the follow-up period with serial MRI scans.
Conclusion
CNs may present with atypical features in a location with a usual histopathological findings. To our knowledge, we described the third case of tetraventricular CN, which was partially treated with both surgical resection and radiosurgery.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflicts of interest.
References
- From the radiologic pathology archives: Intraventricular neoplasms: Radiologic-pathologic correlation. Radiographics. 2013;33:21-43.
- [Google Scholar]
- Central neurocytoma.An electron-microscopic study of two cases. Acta Neuropathol. 1982;56:151-6.
- [Google Scholar]
- Central neurocytoma. In: Kleihues P, Cavenee WK, eds. Pathology and Genetics of Tumours of the Nervous System. Lyon, France: IARC; 2000. p. :107-9.
- [Google Scholar]
- Central neurocytoma: Radiological and clinico-pathological findings in 18 patients and one additional MRS case. J Neuroradiol. 2013;40:101-11.
- [Google Scholar]
- Tumors in the temporal horn of the lateral ventricle as a cause of epilepsy. J Child Neurol. 2008;23:315-20.
- [Google Scholar]
- Atypical central neurocytoma of fourth ventricle with hemorrhagic complication during surgery in a child. Clin Neurol Neurosurg. 2012;114:182-4.
- [Google Scholar]
- Treatment of posterior third ventricular central neurocytoma with endoscopic biopsy, endoscopic third ventriculostomy and stereotactic radiosurgery. Minim Invasive Neurosurg. 2003;46:165-8.
- [Google Scholar]
- Central neurocytoma in third and fourth ventricles with aqueductal involvement. Br J Neurosurg. 2006;20:57-62.
- [Google Scholar]
- Giant central neurocytoma with tetraventricular and extra-axial extension. Case report. Acta Neurochir (Wien). 1995;133:95-100.
- [Google Scholar]
- Central neurocytoma: Histopathological variants and therapeutic approaches. J Neurosurg. 1992;76:32-7.
- [Google Scholar]
- Diffusion weighted MR imaging and proton MR spectroscopy findings of central neurocytoma with pathological correlation. J Neuroradiol. 2014;41:243-50.
- [Google Scholar]
- Central neurocytoma: Long-term outcomes of multimodal treatments and management strategies based on 30 years’ experience in a single institute. Neurosurgery. 2013;72:407-13.
- [Google Scholar]






