Translate this page into:
Clinical features and risk factor analysis for lower extremity deep venous thrombosis in Chinese neurosurgical patients
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Deep venous thrombosis (DVT) contributes significantly to the morbidity and mortality of neurosurgical patients; however, no data regarding lower extremity DVT in postoperative Chinese neurosurgical patients have been reported.
Materials and Methods:
From January 2012 to December 2013, 196 patients without preoperative DVT who underwent neurosurgical operations were evaluated by color Doppler ultrasonography and D-dimer level measurements on the 3rd, 7th, and 14th days after surgery. Follow-up clinical data were recorded to determine the incidence of lower extremity DVT in postoperative neurosurgical patients and to analyze related clinical features. First, a single factor analysis, Chi-square test, was used to select statistically significant factors. Then, a multivariate analysis, binary logistic regression analysis, was used to determine risk factors for lower extremity DVT in postoperative neurosurgical patients.
Results:
Lower extremity DVT occurred in 61 patients, and the incidence of DVT was 31.1% in the enrolled Chinese neurosurgical patients. The common symptoms of DVT were limb swelling and lower extremity pain as well as increased soft tissue tension. The common sites of venous involvement were the calf muscle and peroneal and posterior tibial veins. The single factor analysis showed statistically significant differences in DVT risk factors, including age, hypertension, smoking status, operation time, a bedridden or paralyzed state, the presence of a tumor, postoperative dehydration, and glucocorticoid treatment, between the two groups (P < 0.05). The binary logistic regression analysis showed that an age greater than 50 years, hypertension, a bedridden or paralyzed state, the presence of a tumor, and postoperative dehydration were risk factors for lower extremity DVT in postoperative neurosurgical patients.
Conclusions:
Lower extremity DVT was a common complication following craniotomy in the enrolled Chinese neurosurgical patients. Multiple factors were identified as predictive of DVT in neurosurgical patients, including the presence of a tumor, an age greater than 50 years, hypertension, and immobility.
Keywords
Anterior tibial vein
common femoral vein
deep femoral vein
deep venous thrombosis
posterior tibial vein
superficial femoral vein
Introduction
Deep venous thrombosis (DVT) is a venous return disorder caused by abnormal coagulation of blood in the deep venous system and is a common complication after surgery. The main hazard of DVT is acute stage thrombus shedding, which can cause a pulmonary embolism (PE) and acute respiratory distress syndrome when the blood flow blocks the pulmonary artery. An average incidence of DVT of 24 percent was found among 474 untreated control neurosurgical patients.[1] However, based on the current literature, the incidence of DVT varies in patients with different diseases. For example, the incidence of DVT is 1.5–18% and 32% in patients with a subarachnoid hemorrhage and in patients with a brain tumor, respectively.[23] In addition, the incidence of DVT after craniotomy has been reported to be as high as 50%, and using a threshold of 2 mg/L, D-dimer levels indicate venous thromboembolism with a high degree of sensitivity and specificity in patients who have undergone craniotomy.[4] The present study prospectively investigated the DVT incidence of neurosurgical patients after surgery and analyzed the main risk factor of DVT to help avoid this lethal complication in the future.
Materials and Methods
Study objective
After approval by the Zhengzhou University's Ethics Committee, a single-center prospective study was carried out at the First Hospital Affiliated of Zhengzhou University from July 2012 to December 2013. The study included 196 patients who underwent surgical treatment at our hospital. Of the196 patients, 97 were male (49.5%) and 99 were female (50.5%) and their age ranged from 10 to 84 years. The mean age was 54.24 ± 14.07 years. There were 61 cases of lower extremity DVT after surgery, including 31 male (50.8%) and 30 female (49.2%) patients who ranged from 21 to 83 years of age, with a mean of 55.38 ± 13.21 years. Lower extremity DVT was excluded before surgery for all patients by color Doppler ultrasound examination, and no patients had a history of DVT. Postoperative lower extremity DVT was treated with nursing measures in all cases and medications and interventional therapy in some cases. The nursing measures included affected limb immobilization, elevation, and avoidance of kneading, etc. For the medications, low molecular weight heparin anticoagulant therapy (once daily subcutaneous injections of 4000 IU of low molecular weight heparin with calcium) was administered in 18 cases (29.5%) to promote blood circulation to remove blood stasis, and plasma volume expansion treatment (vinpocetine, low molecular weight dextran, etc.,) was administered in 40 cases (65.6%). While medications were being administered coagulation and platelet functions were monitored. The interventional therapy of inferior vena cava filter placement was performed in 3 cases (4.9%).
Deep venous thrombosis diagnostic criteria
According to the Diagnosis and Treatment Guide of DVT created previously.[56] The diagnosis of DVT should be confirmed through auxiliary examinations, including Doppler ultrasound, plasma D-dimer, confidential interval venography, magnetic resonance imaging venography and angiography, etc. The Doppler ultrasound diagnostic points of DVT are as follows: (1) The probe pressurized venous lumen is not completely closed; (2) the diameter of the embolization segment vein widens obviously, and the thrombosis echo within the lumen has varying degrees of intensity; (3) color Doppler ultrasound provides color flow imaging during embolization that indicates that the vein is thinned or that there is no blood flow; (4) pulse Doppler shows no blood spectrum in the thrombus segment, and the blood spectrum of the distal thrombus does not change with respiration; and (5) Valsalva test is abnormal.
Research methods
Lower extremity color Doppler ultrasound examinations and plasma D-dimer determinations were carried out in all selected patients on days 3, 7, and 14 after the operation. Color Doppler ultrasound assessment was performed using a Toshiba Aplio XG color Doppler ultrasound diagnostic apparatus, which has a probe frequency of 3–12 MHz, to examine the following deep veins of the lower extremities: Common femoral vein (CFV), superficial femoral vein (SFV), deep femoral vein (DFV), popliteal vein (PV), anterior tibial vein (ATV), posterior tibial vein (PTV), peroneal vein (PEV), and calf muscle vein (CMV). Plasma D-dimer examinations were performed using a Beckman ACL TOP 700-type automatic coagulation analyzer and the immune turbidimetry method, with reference values of 0–0.5 mg/L.
Data collection
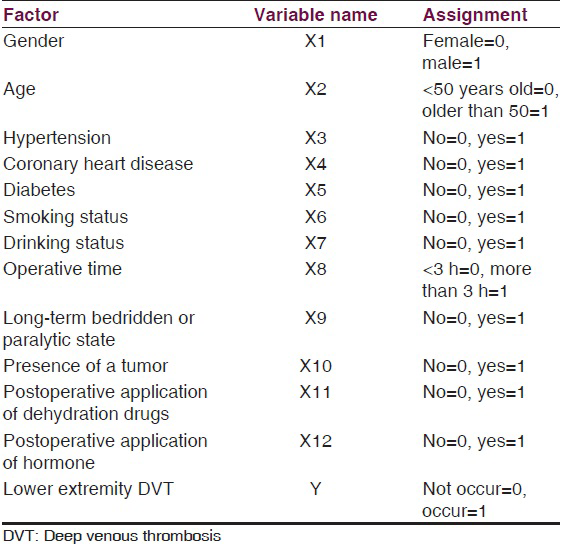
The patients were divided into a thrombus and control group based on whether they experienced a DVT. For this study, the occurrence or absence of lower extremity DVT was the dependent variable, and gender, age, hypertension, coronary heart disease, diabetes, smoking status, drinking status, operative time, a long-term bedridden or paralyzed state, the presence of a tumor, and the application of dehydration drugs and hormones after surgery were independent variables that may have affected the incidence of postoperative DVT in these neurosurgical patients [Table 1].
Statistical methods
The data are presented as the means ± standard deviations (x̄± s), and comparisons between two groups were performed using the t-test with statistically significant differences being indicated by a P < 0.05. The risk factor analysis involved a Chi-square test for the univariate analysis to screen for factors with statistical significance, and then a dichotomous multivariate logistic regression analysis for the multivariate analysis. For both the univariate and multivariate analyses, statistically significant differences were indicated by a P < 0.05. This was used to identify risk factors of lower extremity DVT after neurosurgery.
Results
Clinical symptoms and signs of deep venous thrombosis
In this study, 61 patients (31.1%) experienced lower extremity DVT after surgery, including 37 (60.7%) asymptomatic and 24 symptomatic cases (39.3%). The most common symptom was limb swelling (20 cases, 83.3%) [Figure 1], followed by lower limb pain (16 cases, 66.7%); and then increased local soft tissue tension (11 cases 45.7%). Seven patients (29.2%) experienced all three of these symptoms simultaneously. A few patients had other DVT symptoms, such as a change in skin color, an increase in muscular tension, superficial vein distension, etc. Only 12 patients (50%) exhibited clinical signs of DVT, either Homan's sign or Neuhof's sign.

- Preoperative contrast-enhanced T1MRI showing tuberculum sellae meningioma on axial (a) and sagittal (b) planes; postoperative contrast-enhanced T1MRI showing complete resection at 3 days after operation on axial (c) and sagittal (d) planes. The left lower extremity swelling occurred at 7 days (e), and color Doppler ultrasound detected deep venous thrombosis in common femoral vein, superficial vein and deep femoral vein (f)
The distribution of deep venous thrombosis
For the 61 lower extremity DVT patients, the color Doppler ultrasound results showed that 38 patients had a thrombus in the left lower limb (62.3%), 14 had a thrombus in the right lower limb (23.0%), and 9 had thrombi in both lower limbs (14.8%). The affected veins were as follows: CFV, 2 cases (3.3%); SFV, 5 cases (8.2%); DFV, 3 cases (4.9%); PV, 6 cases (9.8%); ATV, 4 cases (6.6%); PTV, 11 cases (18.0%); PEV, 24 cases (39.3%); and CMV, 52 cases (85.2%) [Figure 2].

- Bar graph showing the vein segment involved in deep venous thrombosis after craniotomy
The univariate and multivariate analyses of factors affecting deep venous thrombosis after neurosurgery
The univariate analysis results showed statistically significant differences between the thrombus group and control group in age, hypertension, smoking status, operative time, a long-term bedridden or paralyzed state, the presence of a tumor, the postoperative application of dehydration drugs and hormones, and other factors (P < 0.05) [Table 2]. The multivariate analysis and dichotomous multivariate logistic regression analysis were performed on the above factors, and we concluded that an age greater than 50 years, hypertension, a long-term bedridden or paralyzed state, the presence of a tumor, and the postoperative application of dehydration drugs are risk factors for lower extremity DVT after neurosurgery [Table 3].


Analysis of plasma D-dimer detection values
The plasma D-dimer detection values of the thrombus group before surgery and at 3, 7, and 14 days after surgery were (0.34 ± 0.11), (5.73 ± 2.19), (3.91 ± 2.01), and (2.37 ± 1.18) mg/L, respectively. Those values for the control group at the corresponding times were (0.26 ± 0.19), (3.92 ± 1.05), (1.36 ± 0.92), and (1.14 ± 0.76) mg/L, respectively (P < 0.05) [Table 4].

Discussion
In neurosurgical patients, lower extremity DVT is a life-threatening complication. Taniguchi et al. reported an incidence of PE in neurosurgical patients with DVT of 60%;[7] moreover, DVT may lead to a PE, which is lethal in up to 50% of affected neurosurgical patients.[89] The rate of lower extremity DVT after neurosurgery was 31.1% in our series. In the present study, there are several striking clinical features obtained from our series of 196 consecutive patients: (1) 60.7% of lower extremity DVTs were asymptomatic and only 39.3% of DVTs were symptomatic. (2) The most common DVT symptom was swelling of the affected limb. In addition, pain and increasing soft tissue tension were other common clinical symptoms of lower extremity DVT after neurosurgery. (3) The main signs of lower extremity DVT include Homan's sign and Neuhof's sign. (4) The most common age of patients involved in DVT ranged from 51 to 60 years. (5) There were no pulmonary emboli or cases of sudden death after administering aggressive treatment.
There are many reports of a higher incidence rate of left lower extremity DVT than right lower extremity DVT.[10] In this study, the side distribution of lower extremity thrombosis was consistent with previous reports; for 61 lower extremity DVT patients, the locations of the DVTs were predominantly in the left lower extremity. In addition, we found that the thrombosis position of neurosurgical patients, who had postoperative lower extremity DVT with paralysis, almost exclusively occurred in paralyzed limbs, and acroparalysis is associated with blood flow slowdown and blood stasis. We also found that the most common sites of venous involvement were the calf muscular vein, PEV and PTV. After analyzing 317 patients with DVT, Kamphuisen and Agnelli[11] found that 60% of patients had calf venous thrombosis, and DVTs were most commonly located in the gastrocnemius venous plexus (38.5%). In our study, the most commonly affected veins of the lower extremity DVT patients were located in the distal lower extremities, predominantly the calf muscular venous plexus. This occurred because venous blood flows slowly in the distal lower extremities; thus, factors, such as braking in bed, cause the muscle pump function to weaken or disappear, which can easily result in calf venous blood stasis and DVT.
In a study of 443 patients receiving craniotomy, Macdonald et al.[12] identified the following risk factors of postoperative lower extremity DVT: Postoperative limb paralysis, long-term Intensive Care Unit treatment, prolonged hospitalization, a long-term postoperative bedridden state, a delay in returning to activity, etc. In our study, the multivariate logistic regression analysis showed that an age >50 years is a risk factor of postoperative lower extremity DVT for neurosurgical patients, because the increase in underlying diseases and the decrease in exercise also increase the incidence rate of DVT.[2] Another risk factor for the development of lower extremity DVT is hypertension, which is an increase in vessel wall pressure leading to angiosclerosis and intima injury. A long-term bedridden or paralyzed state is another major risk factor for DVT after neurosurgery that was found in the present series. In this study, among 61 patients with postoperative lower extremity DVT, 48 (78.7%) were in a long-term bedridden or paralyzed state. Lower extremity paralysis can weaken the squeezing action of muscle contraction on deep veins; this hinders venous return and generates blood stasis.[13] In our practice, some neurosurgical patients may involve frontal sinus opening or traumatic skull base fractures, which require long-term bed rest to prevent cerebrospinal fluid leakage; this makes it easy for limb muscle strength weakness and paralysis to occur. Consequently, the DVT risk factor of long-term bedridden or paralyzed states needs to be given adequate attention. Additionally, the presence of a tumor was another important risk factor for the occurrence of lower extremity DVT after neurosurgery. Many studies have shown found that the incidence rate of DVT and PE in patients with brain tumors is higher than for other neurosurgical diseases,[14] the reasons is due to an imbalance in coagulation and anticoagulation systems, increasing the risk of lower extremity DVT.[15] Specifically, brain tumors have been found to inhibit plasmin release, increase thromboplastin release, increase platelet aggregation, and enhance coagulation.[16]
In addition to the above risk factors of lower extremity DVT after neurosurgery observed in this study, there were some other factors that may be associated with the occurrence of DVT. Smoking can oxidize low-density lipoproteins, increase blood viscosity, and promote platelet activation.[17] In the univariate analysis of this study, smoking status was significantly different between the thrombus group and the control group; however, this difference was not significant by the multivariate regression analysis. Second, gestation may be a risk factor of DVT. This study population included 2 patients that had undergone a cesarean section; they received operative treatment due to traumatic brain injury and glioma, respectively, and both experienced DVT after surgery. This may be associated with the increase of coagulation factors during pregnancy, and related mechanisms require further study. Third, hyperlipemia may be a risk factor of DVT, because significantly higher blood lipid levels have been reported in DVT patients as compared to a normal group.[7] Fourth, infection is another possible risk factor for DVT. Infection can directly damage the vascular intima and also can release a variety of inflammatory mediators, which can activate the extrinsic coagulation system, causing coagulation abnormalities and promoting the development of DVT.[18]
Previous studies have shown excessive D-dimer level was considered to be a risk factor of thromboembolic disease after spinal surgery.[1920] The present study found that D-dimer level was closely associated with the development of DVT after craniotomy. The peak plasma D-dimer level occurred on the 3rd day after craniotomy, and the mean plasma D-dimer level gradually decreased from 3 to 14 days after surgery. This phenomena was consistent with the timing of development of DVT, because 65.6% of DVTs were detected within 1 week after surgery; however, whether D-dimer level can be used as an indicator of DVT requires further exploration in future.
Financial support and sponsorship
This study was supported partially by the Natural Scientific Foundation of China (U1204807).
Conflicts of interest
There are no conflicts of interest.
References
- Enoxaparin plus compression stockings compared with compression stockings alone in the prevention of venous thromboembolism after elective neurosurgery. N Engl J Med. 1998;339:80-5.
- [Google Scholar]
- D-dimer plasma level: A reliable marker for venous thromboembolism after elective craniotomy. J Neurosurg. 2013;119:1340-6.
- [Google Scholar]
- Incidence of deep venous thrombosis after subarachnoid hemorrhage. J Neurosurg. 2009;110:1010-4.
- [Google Scholar]
- The incidence of heparin-induced thrombocytopenia Type II in patients with subarachnoid hemorrhage treated with heparin versus enoxaparin. J Neurosurg. 2009;110:50-7.
- [Google Scholar]
- The utility of screening for deep venous thrombosis in asymptomatic, non-ambulatory neurosurgical patients. J Vasc Surg. 2011;53:1309-15.
- [Google Scholar]
- A National Clinical Guideline Scottish Intercollegiate Guidelines Network (SIGN). Prevention and management of venous thromboembolism. A national clinical guideline. Edinburgh: Scottish Intercollegiate Guidelines Network (SIGN); 2010. p. :1-101.
- [Google Scholar]
- Prevalence of venous thromboembolism in neurosurgical patients. Heart Vessels. 2009;24:425-8.
- [Google Scholar]
- Venous thromboembolism: Deep venous thrombosis and pulmonary embolism in a neurosurgical population. J Neurosurg. 2011;114:40-6.
- [Google Scholar]
- Venous thromboembolism in neurosurgery and neurology patients: A review. Neurosurgery. 1994;34:280-96.
- [Google Scholar]
- The anatomy of deep venous thrombosis of the lower extremity. J Vasc Surg. 2000;31:895-900.
- [Google Scholar]
- What is the optimal pharmacological prophylaxis for the prevention of deep-vein thrombosis and pulmonary embolism in patients with acute ischemic stroke? Thromb Res. 2007;119:265-74.
- [Google Scholar]
- Safety of perioperative subcutaneous heparin for prophylaxis of venous thromboembolism in patients undergoing craniotomy. Neurosurgery. 1999;45:245-51.
- [Google Scholar]
- Mechanical compression in the prophylaxis of venous thromboembolism. ANZ J Surg. 2007;77:418-23.
- [Google Scholar]
- Postoperative venous thromboembolism and brain tumors: Part I. Clinical profile. J Neurooncol. 1992;14:119-25.
- [Google Scholar]
- Venous thromboembolism occurs frequently in patients undergoing brain tumor surgery despite prophylaxis. J Thromb Thrombolysis. 1999;8:139-42.
- [Google Scholar]
- The influence of smoking and of oral and transdermal nicotine on blood pressure, and haematology and coagulation indices. Thromb Haemost. 1997;78:1093-6.
- [Google Scholar]
- Phylogenetic and functional relationships between coagulation and the innate immune response. Crit Care Med. 2000;28(9 Suppl):S77-80.
- [Google Scholar]
- Correlation analysis on plasma D-dimer level with deep venous thrombosis after spinal surgery. Zhongguo Gu Shang. 2014;27:405-8.
- [Google Scholar]
- Analysis of measured D-dimer levels for detection of deep venous thrombosis and pulmonary embolism after spinal surgery. J Spinal Disord Tech. 2011;24:E35-9.
- [Google Scholar]






