Translate this page into:
Totally thrombosed giant anterior communicating artery aneurysm
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Giant anterior communicating artery aneurysms are rare. Apatient presented with visual dysfunction, gait ataxia and urinary incontinence. MRI showed a giant suprasellar mass. At surgery, the lesion was identified as being an aneurysm arising from the anterior communicating artery. The difficulty in preoperative diagnosis and relevant literature are reviewed.
Keywords
Anterior communicating artery
aspect ratio
cavernoma
giant aneurysm
intra-luminal thrombus
pterional
Introduction
Giant intracranial aneurysms have a lower propensity to bleed than small and medium-sized lesions and they may present with symptoms secondary to mass effect. While the diagnosis of a giant aneurysm can be readily made on radiology if there is any circulating component within the aneurysmal sac, establishing a pre-operative diagnosis becomes difficult if the aneurysm is completely thrombosed. The absence of a circulating component and the visualization of a large thrombus would lead to consideration of various other differentials depending on the location of the lesion.
Case Report
A 45-year-old male presented with complaints of progressivelyworsening vision in his left eye and headache since 6 months. He also complained of gait ataxia and occasional social incontinence of urine. On examination, visual acuity in the left eye was 6/36. Fundoscopy revealed mild pallor of the optic disc. He had no other neurologic deficits. MRI brain revealed a large left basifrontallesion extending superiorly and distorting the corpus callosum [Figure 1]. The lesion was hyperintense on T1-weighted images (WI) [Figure 1a] and hypointenseon T2WI[Figure 1b]. The lesion did not enhance on Gd-contrast sequences and no blood flow was demonstrated within the lesion on contrast MR angiography (MRA). CT angiography (CTA) demonstrated displacement of left anterior and middle cerebral arteries; there was no enhancement within the lesion [Figure 1d]. The hormone profile was normal.
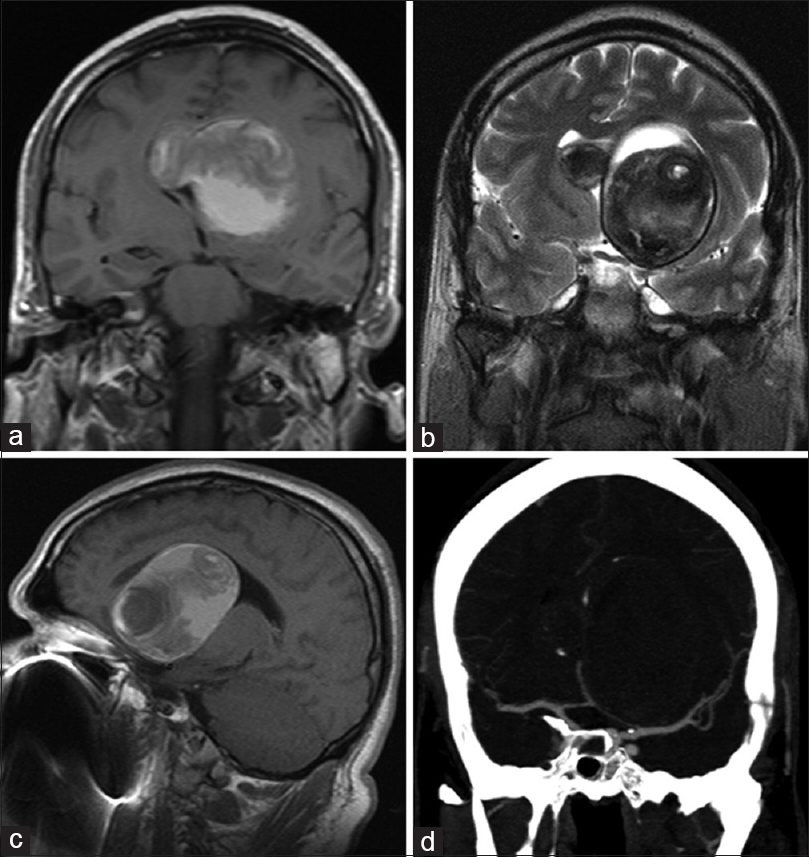
- Preop radiology. (a) T1-weighted coronal image showing a T1 hyperintense lesion. (b) T2WI demonstrating the hyppointense capsule as well as contents. (c) Sagittal T1WI demonstrating the distortion of the corpus callosum. (d) CT angiogram demonstrating the distortion of the left MCA and ACAs
Based on the location and presence of a large thrombus, adifferential of giant cavernous hemangioma was considered. However, the MRI appearance of a well-delineated sac with a thrombus within it was not entirely concordant with this diagnosis. The possibility of the lesion being a giant aneurysm was also considered. However, digital subtraction angiogram (DSA) did not reveal any filling of contrast within the sac of the lesion. The DSA also revealed that the left middle cerebral artery was displaced inferiorly by the lesion [Figure 2a]. The left A1 was not well visualized; both A2s were filling on right carotid injection and a round shift of the A2s was noted [Figure 2b]. Since DSA did not demonstrate any contrast within the lesion, a possibility of intracerebral intravascular papillary endothelial hyperplasia (Masson's tumor) was also considered, although this is a rare lesion within the brain.

- Preop DSA. (a) Right carotid injection—both A2s are filling from the left A1. The A2s are shifted to the right due to the mass effect of the lesion. (b) Left carotid injection. The left MCA is displaced inferiorly by the lesion. No contrast is seen within the lesion
A left pterional craniotomy was made and the lesion approached via the trans-Sylvianroute. Per-operatively, the lesion was found to have a thick capsule. On opening the capsule, a multilayered thrombus was found and evacuated. The lesion wasrelatively avascular and following internal decompression, thecapsule was dissected all around. There was a good plane of cleavage and capsular resection was performed in a piecemeal manner. The optic nerve was then dissected off the capsule. However, a portion of the capsule was found to be significantlyadherent to the left A1-A2 junction. There were areas of calcification in this area that further rendered dissection from the A1-A2 complex difficult [Figure 3a]. There was no plane of cleavage that permitted dissection and excision of the capsule. Multiple perforators were found arising from the A1–A2 junction and traversing posteriorly; these were adherent to the capsule. Hence, that portion of capsule was left behind. Postoperative contrast-enhanced CT and MRI revealed no residual capsule[Figure 3b] and subsequent DSA revealed preserved flow in all vessels and no fresh abnormality [Figure 3c]. Histopathology of the resected specimen was diagnostic of aneurysmal wall with thrombus [Figure 3d]. Sixweeks after surgery, visual acuity in his left eye had improved to normal. Follow-up DSA is planned to ensure that no regrowth of the aneurysm has occurred.

- (a) Intraoperative image showing the optic chiasm (star); the lesion (horizontal arrow) is closely adherent to the left A1 (vertical arrow). The ACom complex is hidden by the lesion in this image. (b) Postop T1 sagittal image demonstrating the complete excision of the lesion. (c) Postop angiogram demonstrating the right internal carotid artery supplying both A2 arteries across a patent AComA. (d) Photomicrograph of the aneurysm wall composed of fibrocollagenous wall, congested blood vessels, lymphocytic infiltrate and hemosiderin laden macrophages (H and E ×400)
Discussion
Spontaneousand complete thrombosis of giant intracranial aneurysm is reported to occur in 3-20% of cases.[1] The majority of giant aneurysms that have been reported to have undergone spontaneous thrombosis arose from the cavernous segment of the internal carotid artery.[2] There are singlecase reports of giant posterior cerebral artery, superior cerebellar artery and pericallosal artery aneurysmsthat underwent spontaneous thrombosis.[3] Such an occurrence appears to be anecdotally rare.
There exist no previous reports of giant anterior communicating artery aneurysms undergoing spontaneous thrombosis. In the present instance, aneurysm was not considered as the top preop differential since the lesion did not enhance on contract administration. Intra-operatively, the only clues to the nature of the lesion werethe presence of a multi-lamellatedthrombuswithin the cavity and the attachment to the anterior communicating artery. The area of attachment (neck) had areas of calcification. It was only the histopathology report that confirmed the diagnosis of giant thrombosed aneurysm. Totally thrombosed giant aneurysms can be confused with other tumors like cavernous hemangiomas, Masson's tumor, oligodendrogliomas and occasionally meningiomas.
There are several hypotheses to explain the phenomenon of spontaneous thrombosis in giant intracranial aneurysms. The fundus of a giant aneurysm may itself compress the parent artery, resulting in stenosis and a low-flow state. This may lead to intra-aneurysmal clot formation ultimately resulting in thrombosis of the aneurysm.[2] A large aspect ratio (indicative of a small neck) could also result in a low-flow state. Extension of the thrombus to the neckwould result in a further reduction of blood flow within the sac resulting in total thrombosis. It is possible that in the present case similar mechanisms could have been responsible for complete isolation of the aneurysmal sac from the circulation. The formation of a neo-intimal layer in the narrow neckcould explain the absence of bleedingdespite close dissection of the sac from the A1–A2 junction.
Pre-operative DSA is an absolute imperative to establish the diagnosis of large thrombosed intracranial mass lesions. Most giant aneurysms retain a circulating component that will be visible on DSA. Totally thrombosed aneurysms such as the present case are extremely rare. This lesion was not recognized as an aneurysm at surgery owing to the complete absence of bleeding and the calcification at the neck; thus, the neck was not clipped. Areas of atherosclerosis and calcification may sometimes preclude effective clipping. However, it is generally recommended that a clip be placed across the neck since there have been several reports of regrowth of giant aneurysms that have not been clipped and completely excluded from circulation. Regular post-op imaging is planned in the present case.
Conclusions
Spontaneous thrombosis of giant intracranial aneurysms is a rare phenomenon. But aneurysms need to be considered in the differential diagnosis of mass lesions located in close proximityto cerebral vasculature. The aim of surgery would be to decompress the surrounding neural structures. A clip application across the neck may not be necessary if there is no filling of the sac while dissecting close to the neck.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Spontaneousthrombosis in giant intracranial aneurysms. J NeurolNeurosurgPsychiatr. 1982;45:1040-7.
- [Google Scholar]
- Two cases of spontaneous occlusion of the internal carotid artery due to a giant intracranial internal carotid artery aneurysm. No ShinkeiGeka. 1988;16:211-5.
- [Google Scholar]
- Spontaneous total thrombosis of distal superior cerebellar artery aneurysm. ActaNeurochir (Wien). 2001;143:837-43.
- [Google Scholar]






