Translate this page into:
Intraventricular racemose type neurocysticercosis with anterior interhemispheric fissure cyst: A rare case report
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Racemose type of neurocysticercosis (NCC) is a rare form of parasitic infestation of central nervous system. Most commonly it is found in fourth ventricle and cisterns. On reviewing the PubMed and Google databases, we found that this would be the first reported case of racemose type NCC in occipital horn of lateral ventricle with obstructive hydrocephalus, along with an incidental interhemispheric fissure arachnoid cyst.
Keywords
Hydrocephalus
interhemispheric fissure arachnoid cyst
neurocysticercosis
racemose
Introduction
Racemose variety of cysticercosis is an uncommon type of neurocysticercosis (NCC), characterized by involvement of ventricular system and subarachnoid cisternal spaces.[12] Intraventricular cysticercosis is a potentially life-threatening entity due to risk of acute obstructive hydrocephalus, either from cyst itself or arachnoiditis induced by NCC, emphasizing the need for an early diagnosis.[3] NCC is caused by cysticercus cellulosae but racemose variety is caused by cysticercus racemosus.[123] Interhemispheric fissure cysts are rare lesions, often associated with complex brain malformations such as corpus callosal agenesis and hydrocephalus.[4] The superior extension of the roof of third ventricle in interhemispheric fissure gives the origin to interhemispheric fissure cysts, which may or may not have communication with third ventricle.[4]
Case Report
A 35-year old female patient admitted with history of gradually progressive headache, vomiting, blurring of vision for last 10 days and loss of consciousness for last 3 days. On examination, patient was unconscious, GCS was 7 (E1V1M5) with left-sided hemiparesis. On fundus examination, bilateral papilledema was present. There were no signs of meningitis. Non-contrast CT head showed anterior interhemispheric fissure cyst with obstructive hydrocephalus.
Contrast MRI showed presence of anterior interhemispheric fissure cyst with agenesis of genu and anterior aspect of body of corpus callosum, agenesis of anterior aspect of interventricular septum with agenesis of septum pellucidum [Figure 1a and e]. Gross dilatation of both lateral ventricles and third ventricle was present [Figure 1d]. Third ventricle was high riding and cerebral aqueduct was also dilated [Figure 1b and g]. The fourth ventricle was not visualized due to obstruction at the roof of ventricle by arachnoiditis [Figure 1c, d and h]. Fluid Attenuation Inversion Recovery (FLAIR) sequence MRI [Figure 2] showed multiple cystic lesions of varying sizes in occipital horn of right lateral ventricle. The Cyst wall was isointense to CSF therefore not visible on T1 and T2 -weighted sequence of MRI. On contrast administration, no contrast enhancement in cyst wall was noted. Scolex was also not visible inside the lesion. No other focal brain parenchymal lesion noted.
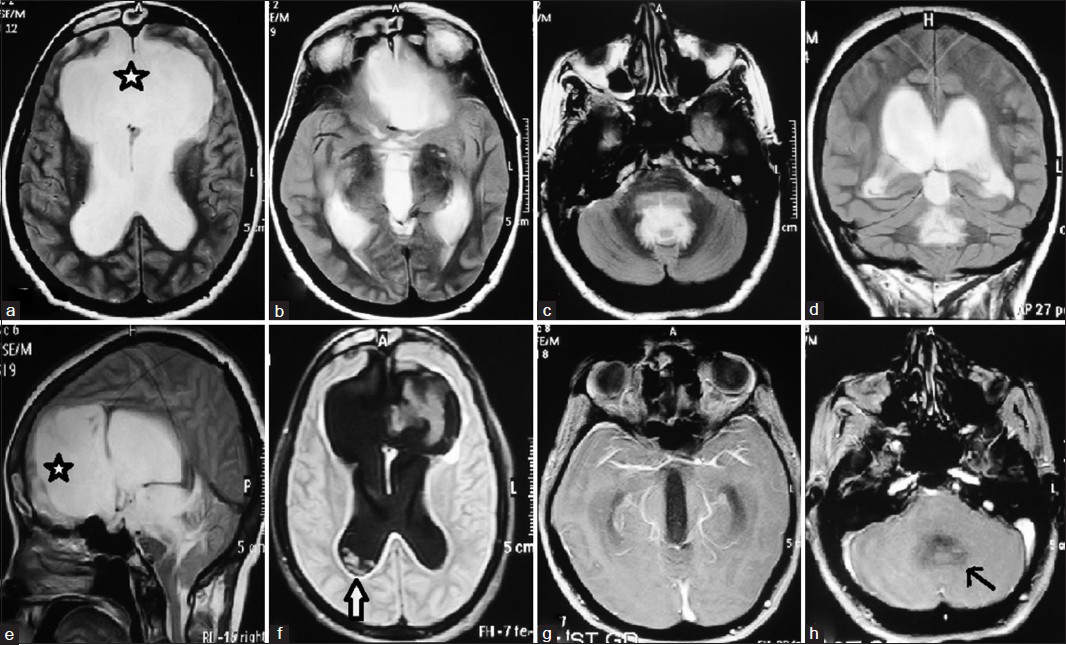
- (a-h) Presence of anterior interhemispheric fissure cyst (a and e) with corpus callosal agenesis, obstructive hydrocephalus due to arachnoiditis at the roof of fourth ventricle (c and h), eith dilatation of both lateral ventricles and third ventricle with non-visualisation of fourth ventricle, with racemose type neurocysticercosis in right occipital horn (f)

- FLAIR sequence MRI showing presence of racemose type neurocysticercosis (open arrow) in right occipital horn of lateral ventricle
Since the patient had deteriorated acutely, left-sided ventriculo-peritoneal shunt was done in emergency. CSF was clear and under high pressure. CSF analysis revealed glucose concentration of 48 mg/dl, protein 35 mg/dl and 20 cells with 40% polymorphs and 60%lymphocytes. CSF-ELISA (enzyme-linked immuno-sorbent assay) was positive for NCC and culture was negative for bacteria, fungi and acid fast bacilli. Our case is fulfilling the criterion of probable diagnosis of NCC by having one major criteria (lesion highly suggestive of NCC on radiological study), one minor criterion (positive CSF-ELISA for NCC) and one epidemiological criterion (India is endemic area for NCC). On postop day 2, patient regained consciousness (GCS = 14). Postoperatively, albendazole (15 mg/kg body weight/day for 4 weeks) and steroids (dexamethasone 0.2 mg/kg body weight/day initially for 5 days and gradually tapered off in next 10 days) given after excluding ocular cysticercosis. The patient was discharged on postoperative day 7. She has been following up regularly with good recovery.
Discussion
NCC is the most common parasitic disease of the central nervous system and the most common cause of acquired epilepsy worldwide.[56] Brain parenchymal involvement occurs in 60% to 92% of patients with NCC, but intraventricular lesions are seen in only 7 to 20% of cases, out of which the lesions are more commonly seen in the 4th ventricle (54-64%), followed by the 3rd ventricle (23-27%), the lateral ventricles (11-14%) and Sylvian aqueduct (9%).[235] Among the reported cases of racemose NCC in lateral ventricle, most of them were confined to temporal horn only and this is the first case of racemose NCC found within occipital horn of lateral ventricle. Ocular and spinal NCC also occurs, but is less common. Spinal cord involvement in NCC is rare, accounting for 1-5% of all cases.[13]
In young age group, development of hydrocephalus due to interhemispheric fissure cyst has been reported in literature but usually it is due to mass effect of the cyst over the ventricle, producing obstructive hydrocephalus.[4] In our case, interhemispheric fissure cyst is located anteriorly near frontal horns of lateral ventricle and 3rd ventricle. If interhemispheric fissure cyst would have been the cause of obstructive hydrocephalus, the frontal horns and third ventricle would be compressed. In our case, there was dilatation of both lateral and 3rd ventricle with obstruction at the level of roof of 4th ventricle, which cannot be explained by interhemispheric fissure cyst. Furthermore, presence of arachnoiditis at the level of 4th ventricle [Figure 1c, d and h] in T1- and T2 weighted MRI suggests an inflammatory cause of hydrocephalus rather than anterior interhemispheric fissure cyst which is a non-inflammatory lesion.
The cysticercus larva (after embedding itself in the parenchyma) undergoes four stages of evolution: Vesicular, colloidal, granulo-nodular, and nodular- calcified.[25] This evolution does not occur in the intraventricular and the subarachnoid form of NCC. Racemose NCC refers to “aberrant proliferating cestode larvae” that manifest as solitary or multiple unencapsulated bladders, which bud exogenously to form a multilocular cyst, resembling a bunch of grapes.[5] Cysts of the racemose NCC are usually found in suprasellar, sylvian and quadrigeminal cistern.[13] These cysts are nonviable, degenerated, interconnected bladders of different sizes which often lack scolices, and can reach up to large size to exert local mass effect. Because of their location, they can produce hydrocephalus, which is caused by inflammation of meninges with subsequent fibrosis and obstruction.[23] The intraventricular cysts can circulate freely throughout the CSF pathways.
The clinical manifestation of NCC ranges from asymptomatic to life threatening condition. Within the CNS it can affect the parenchyma, subarachnoid space, or intraventricular system.[2] Intracranial hypertension can occur in patients with parenchymal NCC and is termed as cysticercotic encephalitis. Increased intracranial pressure due to hydrocephalus can occur if ependymitis occurs at the level of the cerebral aqueduct.[13] Cerebrovascular complications of NCC include cerebral infarction, transient ischemic attacks and brain hemorrhage. The most common mechanism by which NCC produces cerebrovascular complication is related to cerebral arteritis, mainly in patients with subarachnoid cysticercosis. They can also cause sudden death by producing acute ventricular obstruction, resulting in acute hydrocephalus.[13]
Intraventricular cysts are isodense to CSF on CT Scan.[3] MRI is sensitive in the diagnosis of active NCC and may be useful in evaluating degenerative changes in the parasite. Intraventricular cysts are detected on MRI by mass effect, ventricular obstruction, and detection of a cyst rim and/or CSF flow void adjacent to the rim.[6] Intensity of cysts is similar to that of CSF on both T1-and T2 weighted images. FLAIR sequence of MRI is especially useful in detection of intraventricular NCC.[36] 3D-CISS (constructive interference in steady state) MRI is a high resolution heavily T2- weighted sequence with millimeter thin sections, highly sensitive in identifying features of a cysticercal cyst especially the scolex, cyst wall and fluid.[7]
Two serological tests are available for detection of NCC infestation. The current assay of choice is the electroimmunotransfer blot (EITB) using partially purified antigenic extracts (specificity = 100%, sensitivity = 94-98%).[5] Antigen-ELISA is based on the use of a monoclonal antibody (HP10) that reacts with a carbohydrate epitope found over the surface of live cysticerci (specificity = 96%, sensitivity = 86%).[5]
Del Brutto[8] has proposed a set of diagnostic criteria in 2001 to avoid the over diagnosis of NCC that occurs in epidemiologic surveys and to help clinicians evaluating patients with suspected NCC. The set included four stratified categories of criteria, including: (1) Absolute: Histological demonstration of cysticerci, cystic lesions showing the scolex on neuroimaging studies, and direct visualization of subretinal parasites by fundoscopic examination; (2) major:Lesions highly suggestive of NCC on neuroimaging studies, positive serum enzyme-linked immunoelectrotransfer blot (EITB) for the detection of anticysticercal antibodies, resolution of intracranial cystic lesions after cysticidal drug therapy, and spontaneous resolution of single enhancing lesions; (3) minor:Lesions compatible with NCC on neuroimaging studies, suggestive clinical manifestations, positive cerebrospinal fluid (CSF) ELISA for detection of anticysticercal antibodies or cysticercal antigens, and cysticercosis outside the nervous system; and (4) epidemiological: Evidence of a household contact with Taenia solium infection, individuals coming from or living in cysticercosis endemic areas, and history of travel to disease-endemic areas. Interpretation of these criteria permits two degrees of diagnostic certainty: (1) Definitive diagnosis, in patients who have one absolute criterion or in those who have two major plus one minor and one epidemiological criteria; and (2) probable diagnosis, in patients who have one major plus two minor criteria, in those who have one major plus one minor and one epidemiological criteria, and in those who have three minor plus one epidemiological criteria.
Cysticidal drugs with steroids and shunting for hydrocephalus have been used with success as treatment for intraventricular NCC. If hydrocephalus is present, ventriculo-peritoneal shunt should be done prior to medical therapy. Surgery has been the mainstay of treatment in intraventricular NCC. The decision to operate in a viable intraventricular cyst depends on presence of: (i) Mass effect, (ii) CSF obstruction, (iii) fourth ventricular cyst.[23] There is a growing literature supporting flexible neuroendoscopy together with hydraulic maneuver to facilitate the removal of the intact cyst from lateral, third, and fourth ventricle.[12] Albendazole is considered the anti-helminthic drug of choice for NCC because it has better CSF penetration and its concentration is not affected when administered along with steroid.[23] When feasible, complete surgical excision of lesion remains the definitive therapy.[2]
The prognosis for intraventricular NCC is worse than that for the intraparenchymal form of the disease because it usually does not respond to anti-helminthic drug and the rate of shunt dysfunction is also high.[2]
Conclusion
Intraventricular NCC is a rare entity even in endemic areas of cysticercosis. Previously reported cases have shown NCC in temporal horn of lateral ventricle or inside the fourth ventricle. To the best of our knowledge, this is the first case report of intra-ventricular racemose NCC in occipital horn of lateral ventricle with incidental anterior interhemispheric fissure cyst presenting with obstructive hydrocephalus due to cysticercal arachnoiditis.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Endoscopic management of cysticercal cysts within the lateral and third ventricles. J Neurosurg. 2000;92:14-23.
- [Google Scholar]
- Lateral ventricular neurocysticercosis: A case report. Indian J Radiol Imaging. 2006;16:775-8.
- [Google Scholar]
- Imaging features and surgery-related outcomes in intraventricular neurocysticercosis. Neurosurg Focus. 2002;12:6e.
- [Google Scholar]
- Interhemispheric cyst of an adult associated with partial agenesis of the corpus callosum. Rinsho Hoshasen. 1990;35:959-62.
- [Google Scholar]
- Improved detection of intraventricular cysticercal cysts with the use of three-dimensional constructive interference in steady state MR sequences. AJNR Am J Neuroradiol. 2000;21:679-84.
- [Google Scholar]
- Diagnostic criteria for neurocysticercosis, revisited. Pathog Glob Health. 2012;106:299-304.
- [Google Scholar]






