Translate this page into:
Diabetes mellitus and impaired glucose tolerance in patients with schizophrenia, before and after antipsychotic treatment
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Treatment with antipsychotics increases the risk of developing diabetes in patients of schizophrenia but this diabetogenic potential of different antipsychotics seems to be different. Moreover, there may be an independent link between schizophrenia and diabetes. So we plan to study the prevalence of glucose dysregulation in patients of schizophrenia before and after treatment with various antipsychotics.
Materials and Methods:
Fifty patients (32 males and 18 females) diagnosed with schizophrenia were evaluated for glucose dysregulation using oral glucose tolerance test, initially (drug naive) and after antipsychotic treatment. Age- and sex-matched healthy volunteer group of 50 subjects (35 males and 15 females) was taken for comparison. Results were interpreted using American Diabetic Association criteria.
Results:
Though the glycemic status of the patient group was comparable with healthy controls initially but antipsychotic treatment was associated with glucose dysregulation. For first 6 weeks the antipsychotic (olanzapine, risperidone, haloperidol and aripiprazole)-induced glucose dysregulation was comparable, which was seen to be maximum with the olanzapine-treated group at the end of this study, 14 weeks.
Conclusion:
We conclude that antipsychotic treatment of nondiabetic drug naive schizophrenia patients was associated with adverse effects on glucose regulation. For initial 6 weeks the antipsychotic-induced glucose dysregulation was comparable, which was seen to be maximum with olanzapine at the end of study, i.e. 14 weeks. Keeping this at the back of mind we can stabilize a patient initially with a more effective drug, olanzapine, and later on shift to one with less metabolic side effects.
Keywords
Antipsychotic agents
diabetes mellitus
glucose tolerance test
schizophrenia
Introduction
Schizophrenia is a mental disorder characterized by disintegration of thought processes and of emotional responsiveness.[1] Schizophrenia and, indeed, most mental illnesses are associated with undue medical morbidity and mortality.[2] Life expectancy among schizophrenic patients is 20% shorter than in the general population, with circulatory, respiratory, and gastrointestinal illnesses accounting in part for this finding.[3] In addition, patients with schizophrenia also appear to have higher rates of impaired glucose tolerance, insulin resistance, and type II diabetes mellitus than the general population.[4] Rates for diabetes mellitus in patients with schizophrenia are approximately double those reported for the general population.[5]
Diabetes mellitus, or simply diabetes, is a group of metabolic diseases in which a person has high blood sugar, either because the body does not produce enough insulin, or because cells do not respond to the insulin that is produced.[6] Type II diabetes mellitus makes up about 90% of cases of diabetes with the other 10% due primarily to diabetes mellitus type 1 and gestational diabetes. India has around 41 million people with a rate of 3% suffering from type 2 diabetes mellitus. India also leads in prevalence of metabolic syndrome as well as obesity.[6]
Both typical and atypical antipsychotics have been associated with increased risk of diabetes mellitus in patients with schizophrenia. Phenothiazine treatment was observed to contribute to glucoregulatory abnormalities,[67] including reports of aggravation of existing diabetes[8] and new-onset type II diabetes.[91011] Recent reports suggest that newer antipsychotic medications also contribute to clinically significant hyperglycemia. Hyperglycemia, exacerbation of existing diabetes, new-onset type 2 diabetes, and diabetic ketoacidosis have been reported with atypical antipsychotics, especially for clozapine[121314] and olanzapine.[15161718]
An independent link between schizophrenia and glucose dysregulation was suggested by investigators before the advent of neuroleptics. However, these data must be interpreted with caution because the definitions of diabetes and schizophrenia used then differ somewhat from those used today.[19202122] Schizophrenia and diabetes may be linked independently of medication comes from the observation that the rate of type II diabetes mellitus in family members of schizophrenic patients is between 18% and 30%,[23] which is far higher than the rate in the population at large (1.2-6.3%).[24]
Few studies have evaluated glucose dysregulation in schizophrenia in Indian subcontinent and none has been from our Kashmir region, so we planned a study with the aim to evaluate the magnitude of glucose dysregulation in drug-naive, first-episode patients with schizophrenia before and after the commencement of antipsychotic use. A healthy volunteer group matched for age, sex, diet and exercise variables, and various anthropometric measures was used as a control group.
Materials and Methods
Setting
The study was carried out at the outpatient unit of a tertiary care psychiatry Hospital in North India over a period of one and half year, from April 2011 to September 2012 after seeking permission from the ethics committee of the Hospital. The patients were diagnosed with schizophrenia as per diagnostic and statistical manual of mental disorders 4th text revision (DSM IV TR). Informed consent was obtained from each patient, those who were considered incapable of consenting participated with the consent of their closest family member or guardian.
Subjects
First episode, drug naive schizophrenia subjects, 32 males and 18 females, were taken up for the study. All patients were subjected to a baseline oral glucose tolerance test initially before allocating them randomly to four antipsychotic treatment groups. Aripiprazole, Risperidone, Haloperidol and Olanzapine were the four treatment groups. Aripiprazole and Risperidone groups received 13 patients each while Haloperidol and Olanzapine groups received 12 patients each. Follow-up OGTT was recorded at 6 and 14 weeks in each group.
A healthy volunteer group of 50 subjects, 35 males and 15 females, matched for age, sex, diet and exercise variables were taken and subjected to baseline OGTT.
Assessment
OGTT was performed in the morning as glucose tolerance can exhibit a diurnal rhythm with a significant decrease in the afternoon. A zero time (fasting) venous blood sample was drawn. The patient's were then given a 75 g oral glucose load within a 5 minute time frame. Venous blood was drawn after an interval of 2 hours for measurement of glucose (blood sugar). Results of impaired glucose tolerance were interpreted according to American Diabetic Association criteria.[25]
Exclusion criteria
-
Those who do not consent
-
Medical conditions that could confound glucoregulatory assessments including history of diabetes mellitus, cardiovascular, and respiratory conditions with hemodynamic compromise or hypoxia, malignancy, epilepsy, endocrine conditions (excluding corrected thyroid abnormalities), current fever, pregnancy or high dose estrogen therapy, narcotic, corticosteroid or spironolactone therapy, sedative hypnotic withdrawal, any change in medication within 10 days of study.
Statistical analysis
Categorical variables were summarized as percentage while as continuous variables were summarized as mean ± standard deviation. Repeated measures ANOVA followed by a post hoc test (LSD) was used to compare fasting and 2 hr post load plasma glucose levels between different drug groups. Repeated measures ANOVA was used to compare fasting and 2 hr post load plasma glucose levels across follow-up times. A P < 0.005 was taken as significant. Data analysis was done using SPSS version 20.0.
Results
Our sample consisted of two groups. Group I included first episode, drug naive schizophrenia subjects, 32 males and 18 females while group II included the healthy volunteer group of 50 subjects, 35 males and 15 females, matched for age, sex, diet and exercise variables. Other sociodemograpic variables are given in Table 1.
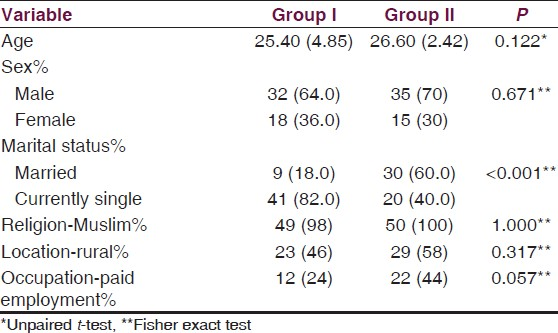
The mean fasting plasma glucose of first episode, drug naïve schizophrenia subjects and healthy controls was 88.34 and 87.40, while post load plasma glucose was 130.26 and 129.98 respectively at baseline in the present study. 18% subjects in the patient group and 4% in the control group where having impaired glucose tolerance as is given in Table 2.

Effect of various antipsychotics on fasting and post glucose load plasma glucose levels on follow up is given in Table 3. When the patients were given antipsychotic medication both fasting and post load plasma glucose showed a continuous increasing trend on follow up. Mean fasting plasma glucose at baseline, 6 weeks and 14 weeks was 88.50, 94.37 and 102.93 while as mean post load plasma glucose was 130.17, 139.89 and 153.02, respectively. Impaired glucose tolerance was found in 18% (n = 9) subjects at baseline which increased to 48% (n = 24) at 6 weeks and 65.2% (n = 30) at the end of 14 weeks.

Table 4 shows the internal comparison of various antipsychotics on follow up. Effect of individual antipsychotic on fasting and post load plasma glucose did not show any significant difference at 6 weeks follow up but there was significant difference at 14 weeks. Pair-wise comparison of fasting and post load plasma glucose at 14 weeks using LSD test showed that the olanzapine group was significantly different from other three groups (aripiprazole, risperidone and haloperidol) while aripiprazole, risperidone and haloperidol groups were similar among themselves.

Discussion
In our study we tried to estimate the abnormalities of glucose regulation in first episode, drug naive patients of schizophrenia before and after antipsychotic treatment in Kashmir Valley. To the best of our knowledge there is no such study in literature from this region (Kashmir Valley) before.
Schizophrenia subjects in our study did not show any significant elevation in plasma glucose when compared with the healthy control group. This finding go against the fact that drug naive schizophrenia patients are two to three times more likely to have type II diabetes than adults in the general population.[26] The lower prevalence of impaired glucose tolerance in first episode, drug naive schizophrenia subjects in the present study could be due to lower mean duration of illness (8 months only) of the study sample.
Antipsychotic treatment of our patient group was associated with impairment in plasma glucose level, which progressed with time. This observation is in accordance with earlier study by Newcomer, et al.;[27] they found that nondiabetic schizophrenia patients showed adverse effects on glucose regulation after they were started on antipsychotic medication, which can vary in severity independent of adiposity and potentially increase long-term cardiovascular risk. Also, Gupta, et al.[28] earlier reported a prevalence rate of 17% for diabetes in a chart review of 208 patients with psychotic disorders who were receiving antipsychotic medication. In our study 52.2% (n = 24) were pre diabetic while 13% (n = 6) were suffering from diabetes mellitus at the end of 14 weeks follow up. Thus, the findings of our study are in accordance with Gupta, et al. (13% vs 17%).
In our study we found that the effect of individual antipsychotic on fasting and post load plasma glucose did not show any significant difference at 6 weeks follow up but there was significant difference at 14 weeks. Pair-wise comparison of fasting and post load plasma glucose at 14 weeks using LSD test showed that the olanzapine-treated patient group had significant plasma glucose elevation in comparison to risperidone, aripiprazole, haloperidol and healthy control groups. Risperidone-, aripiprazole- and haloperidol-treated groups had plasma glucose elevations only in excess to healthy controls while they were comparable among themselves. As we know, typical antipsychotics are characterized by their antagonistic effect and high affinity for dopamine, D2 receptors. Dopamine antagonism is believed to be responsible for their ability to reduce positive symptoms of schizophrenia as well as their tendency to produce EPS. Atypical antipsychotics, in contrast, are antagonists for both serotonin (5-hydroxytryptamine, 5-HT) and dopamine receptors in addition to their effects on H1 and muscarinic receptors. Studies have analyzed relationship between antipsychotic drug affinity for specific receptor subtypes and diabetes. For example, Matsui-Sakata, et al.[29] reported that antipsychotic drug-induced diabetes is directly proportional to affinities for 5-HT2C, H1 and muscarinic receptors. Wirshing, et al.[30] have proposed a serotonergic model for olanzapine-induced diabetes, based on animal models showing that 5-HT1A antagonism decreases pancreatic β-cell responsiveness, thus decreasing insulin secretion and increasing serum glucose levels. Thus, our observation that olanzapine induces more glucose dysregulation than haloperidol (typical antipsychotic) could be explained by its differential receptor occupancy. Risperidone appears to be distinct from olanzapine because it significantly reduces glucose levels through induction of insulin release. This effect could be due to its potent α2 adrenoceptor antagonist activity.[31] Because coadministration of the α2-adrenoceptor antagonist yohimbine with other antipsychotic agents also induces marked insulin release and thus prevents hyperglycemia. Preclinical studies have shown that inadequate compensatory insulin secretion plays a role in olanzapine-induced insulin resistance in animals[32] and that olanzapine and clozapine (3.2-10 mg/kg), but not ziprasidone (32 mg/kg) and risperidone (2 mg/kg), acutely induce profound insulin resistance.[33] Another possible mechanism of difference in glucose dysregulation within the class (atypical antipsychotics) could be differential weight gain. Olanzapine and clozapine cause the greatest, risperidone and ziprasidone moderate while aripiprazole causes least weight gain.[34]
Olanzapine is being now widely used as first-line therapy for schizophrenia, offering efficacy equal to or better than other antipsychotic agents, and has lesser EPS, which is otherwise a stigmatizing and sometimes debilitating side effect for the patient.[3536] However, because of growing evidence of hyperglycemic effects, as is evident from our study also, a prescribing choice must now be based on an assessment of each drug's efficacy as well as its potential to cause movement disorders or metabolic side effects. Moreover, schizophrenia is a chronic illness that requires continuous antipsychotic therapy, an antipsychotic agent with less diabetogenic potential such as aripiprazole and risperidone can be continued to prevent psychotic relapse and long-term deterioration with least effects on glucose dysregulation.
-
Psychiatrists, primary care physicians, and other mental health professionals should be well aware about glucose dysregulation associated with atypical antipsychotics. Before initiating treatment with an antipsychotic agent especially olanzapine, it is imperative to check for any family history of type 2 diabetes mellitus, obtain a baseline weight/BMI, plasma glucose levels and lipid profile. Weight/BMI should be monitored at every visit; plasma glucose levels should be checked at 14 weeks follow-up, as per our observations
-
If a rise in plasma glucose level or weight gain, in a patient on olanzapine, is observed. Switching to low-risk agent, preferably aripiprazole, should be considered and patient monitored for further glucose dysregulation
-
Schizophrenia patients most often than not are potential diabetics, associated with risk factors like sedentary lifestyle, smoking and poor nutrition habits. Such patients should be encouraged to take a low-carbohydrate and high-protein diet with plenty of vegetables. They should also be advised to have at least 30 minutes of physical activity, five times a week[37]
-
We strongly recommend that atypical antipsychotics such as aripiprazole and risperidone, which do not seem to pose a high risk of diabetes, should be considered when initiating drug therapy in potential diabetic patients.
Conclusion
Thus, we conclude that antipsychotic treatment of nondiabetic drug naive schizophrenia patients was associated with adverse effects on glucose regulation. For first 6 weeks the antipsychotic (olanzapine, risperidone, haloperidol and aripiprazole)-induced glucose dysregulation was comparable, which was seen to be maximum with the olanzapine-treated group at the end of this study, i.e. 14 weeks. Awareness of this in clinical practice can have implications in the selection of antipsychotic medication. We can stabilize a patient initially with a more effective drug, olanzapine, and later on shift to one with less metabolic side effects. Timely blood glucose monitoring and advice on healthy lifestyle can reduce the risk of antipsychotic-induced glucose dysregulation.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- “Schizophrenia” Concise Medical Dictionary. In: Oxford United kingdom (8th Edition). Oxford University Press, Maastricht University Library; 2010. p. :411.
- [Google Scholar]
- Preventable Physical Illness in People with Mental Illness. Perth: University of Western Australia; 2001.
- Mortality in a cohort of patients with schizophrenia: A record linkage study. Can J Psychiatry. 1991;36:239-45.
- [Google Scholar]
- Physical consequences of schizophrenia and its treatment, the metabolic syndrome. Life Sci. 2002;71:239-57.
- [Google Scholar]
- Metabolic issues and cardiovascular disease in patients with psychiatric disorders. Am J Med. 2005;118(Suppl 2):15s-22s.
- [Google Scholar]
- Phenothiazine-induced hyperglycemia: Relation to CNS and adrenal effects. Proc Soc Exp Biol Med. 1971;137:1385-8.
- [Google Scholar]
- Hyperglycemia and glycosuria following chlorpromazine therapy. JAMA. 1956;162:1651.
- [Google Scholar]
- Hemolytic anemia, jaundice and diabetes mellitus following chlorpromazine therapy. Can Med Assoc J. 1956;75:746-9.
- [Google Scholar]
- Phenothiazines and diabetes in hospitalized women. Am J Psychiatry. 1968;124:978-82.
- [Google Scholar]
- Prevalence of diabetes and impaired glucose tolerance in patients treated with clozapine compared with patients treated with conventional depot neuroleptic medications. J Clin Psychiatry. 1998;59:294-9.
- [Google Scholar]
- Euglycemic clamp study in clozapine-induced diabetic ketoacidosis. Ann Pharmacother. 2001;35:1381-7.
- [Google Scholar]
- New-onset diabetes mellitus and diabetic ketoacidosis associated with olanzapine treatment. Psychosomatics. 1999;40:438-43.
- [Google Scholar]
- Sugar tolerance in dementia praecox and other mental disorders. Arch Neurol Psychiatry. 1922;8:184-96.
- [Google Scholar]
- Resistance to insulin in mentally disturbed soldiers. Arch Neurol Psychiatry. 1946;56:74-8.
- [Google Scholar]
- The insulin tolerance test in mental disorders. Acta Psychiatr Neurol Scand Suppl. 1952;80:189-99.
- [Google Scholar]
- Vital and Health Statistics. In: Current Estimates from the National Health Interview Survey, 1994. Hyattsville, MD: National Center for Health Statistics; 1995. p. :82-89.
- [Google Scholar]
- Harrison's Principles of Internal Medicine (17th ed). New York: McGraw-Hill Medical; 2008. p. :2275.
- Diabetes and schizophrenia 2005: Are we any closer to understanding the link? J Psychopharmacol. 2005;19(Suppl 6):56-65.
- [Google Scholar]
- Abnormalities in glucose regulation during antipsychotic treatment of schizophrenia. Arch Gen Psychiatry. 2002;59:337-45.
- [Google Scholar]
- Hyperglycemia and hypertriglyceridemia in real world patients on antipsychotic therapy. Am J Ther. 2003;10:348-55.
- [Google Scholar]
- Receptor occupancy-based analysis of the contributions of various receptors to antipsychotics-induced weight gain and diabetes mellitus. Drug Metab Pharmacokinet. 2005;20:368-78.
- [Google Scholar]
- Biochemical profile of risperidone, a new antipsychotic. J Pharmacol Exp Ther. 1988;247:661-70.
- [Google Scholar]
- Metabolic dysregulation with atypical antipsychotics occurs in the absence of underlying disease: A placebo-controlled study of olanzapine and risperidone in dogs. Diabetes. 2005;54:862-71.
- [Google Scholar]
- Acute effects of atypical antipsychotics on whole-body insulin resistance in rats: Implications for adverse metabolic effects. Neuropsychopharmacology. 2007;32:289-97.
- [Google Scholar]
- Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27:596-601.
- [Google Scholar]
- Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209-23.
- [Google Scholar]
- American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27:596-601.
- [Google Scholar]






