Translate this page into:
Malignant nodular hidradenoma of scalp
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Malignant nodular hidradenoma (MNH) is a rare tumor of sweat gland known by many names in the literature. Scalp is a known and yet uncommon site of occurrence. We describe two patients with scalp MNH with brain parenchymal invasion. Both tumors recurred in spite of total excision and radiotherapy.
Keywords
Cutaneous adnexal tumor
malignant nodular hidradenoma
scalp tumor
skull tumor
Introduction
Malignant nodular hidradenoma (MHN) was first reported as clear cell eccrine carcinoma by Keasbey and Hadley in 1954.[1] Several synonyms have been described in the literature, like malignant clear cell myoepithelioma, malignant acrospiroma, clear cell hidradenocarcinoma, clear cell eccrine carcinoma, clear cell hidradenoma, solid-cystic hidradenoma and eccrine acrospiroma.[23] Occurrence in the scalp is rare but known.[4] The natural history of the disease is varied. Wide local excision seems to be the best management strategy.[5] The role of adjuvant therapy is controversial.[5] We describe two cases of MHN managed at our institute.
Case Reports
Case 1
A 14-year-old female patient presented with four year history of progressively increasing swelling of the scalp. At first presentation the swelling was small and appeared as lytic lesion on CT scan [Figure 1]. The patient underwent surgery three times, as swelling recurred frequently. Curettage was done at first time, partial craniectomy second time, and total excision the third time. The duration between first and the second surgery was six months, and between second and third surgery was one year. Histopathology examination showed tumor cells with clear cytoplasm. The cells were arranged in nodules infiltrating the superficial dermis with comedonecrosis. There were frequent mitotic figures [Figure 2]. The findings were suggestive of MNH. The tumor cells were positive for CD99 (membrane positivity) and negative for cytokeratin, desmin, leucocyte common antigen, chromogranin and synaptophysin. The surgical margin was found free from tumor third time. Following the third surgery the patient underwent external beam radiotherapy, 45 Gy in 25 fractions. The patient again developed recurrence within a year. At this time the patient had a massive scalp swelling, which was hard and non tender. CT scan showed a large scalp lesion with streaks of calcification, and brain parenchymal invasion. A surface shaded display reconstruction of CT scan showed sunburst appearance from parietal bone. At this time parents of the patient refused for further treatment.
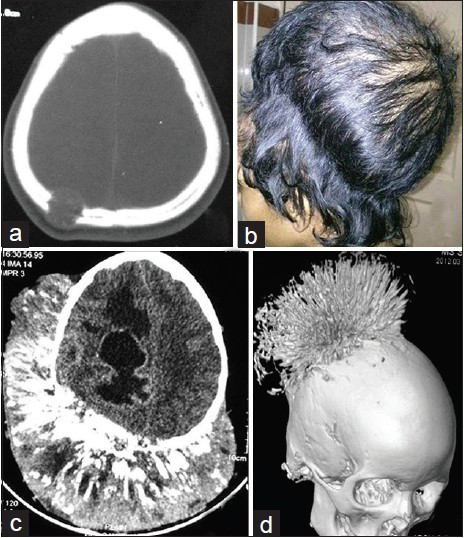
- (a) CT scan at initial presentation showing a lytic lesion in parietal region (b) Latest clinical photograph showing a massive parietal scalp swelling (c) Latest CT scan showing scalp tumor with calcification and brain parenchymal invasion (d) Latest CT scan of head with surface shaded display reconstruction showing tumor involving parietal bone and calcification appearing as sunburst pattern

- Histopathology of tumor resected at time of last recurrence. Hematoxylin and Eosin stain (a) Tumor cells in nodules infiltrating the superficial dermis with comedonecrosis(*). × 50 (b) Tumor cells with clear cytoplasm and comedonecrosis. × 100 (c) Frequent mitotic figures. × 200
Case 2
A 45-year-old female patient had swelling in the left parietal region for three months for which she underwent local excision elsewhere. Within one and a half month the swelling reappeared, and progressed rapidly in next two months. At arrival to our institute she had a large swelling in the left parietal region. CT and MRI scan of the head showed a large scalp swelling causing the bone destruction with intracranial extension [Figure 3]. She underwent left parietal craniotomy and total excision of tumor. Histopathology revealed MNH. She was referred for adjuvant therapy to cancer hospital. She succumbed to her illness while receiving radiotherapy within three months of the surgery.

- (a) MRI showing parietal scalp tumor with brain invasion (b) CT scan showing parietal scalp tumor with bone destruction
Discussion
Sweat gland tumors are mostly benign. Histologically, sweat glands may be either eccrine or apocrine in nature. Eccrine glands are present throughout the skin but are most abundant in the palms, soles, and axillae. Apocrine glands are found in relatively fewer regions of the body, mainly the axillae, around the nipples, the anogenital region, and occasionally a small number on the abdomen and chest.[6] The MNH is an uncommon malignant cutaneous adnexal tumor that can show differentiation toward various components of eccrine sweat glands. It is difficult to estimate the incidence and exact number of cases reported, as MNH is described by varied names in the literature.
The histology of MNH is similar to that of benign nodular hidradenoma.[7] Establishing histopathologic criteria of malignancy for these tumors can be difficult because nuclear anaplasia may be absent, slight, or moderate.[8] The MNH displays an infiltrative or invasive pattern, atypical mitosis, necrosis and angiolymphatic invasion. There are nodular or lobulated architecture of clear cells with glycogen containing cytoplasm and tubular or ductal structures. There may be a large or small representation of cells showing squamoid differentiation. However a diversity of cell types, such as polygonal cells, clear cells and spindle cells may be seen.[5] Most of these tumors begin as small solitary intradermal nodules. A detailed immunohistochemical study of a series of MNH was performed to establish criteria of MNH.[8] Six cases of MNH were evaluated histologically and immunochemically. No consistent pattern was observed when neoplasms were stained with antibodies to carcinoembryonic antigen, S100 protein, gross cystic disease fluid protein 15, epithelial membrane antigen, BCL1, or BCL2. All tumors stained positively for keratin AE1/3 and cytokeratin 5/6, whereas Ki-67 and p53 staining was strongly positive in 5 of the 6 cases (83%). The authors concluded that Ki-67 and p53 staining may be useful histologic parameters. In addition, it may not be necessary to segregate hidradenocarcinomas into eccrine and apocrine categories.[8] The treatment of MNH is not satisfactory. Even after the complete excision MNH have potential for uncontrollable local recurrence, tend to metastasize, and often cause death.[7] About 50-60% patients present with local recurrence or metastasis despite complete removal.[7] In a report of seven cases, six patients died within 15-45 months of diagnosis. The survival time was inversely proportional to the size of tumor.[5] The preferred treatment is early wide surgical excision of the tumor. The efficiency of adjuvant therapy, including the hormonal therapy, is not established.
Conclusion
Malignant nodular hidradenoma is a rare aggressive cutaneous adnexal tumor. Early treatment should be contemplated in all. Wide surgical excision of the tumor with a least 2 cm of clear margins for both primary disease and local recurrences is the treatment of choice. Elective regional lymphadenectomy after lymphoscintigraphy should also be performed. The role of sentinel lymph node biopsy in the treatment of malignant hidradenoma is controversial. Radiotherapy may also be considered though its role needs to be substantiated further.[9] As early recurrence is common despite the wide excision close follow-up is mandatory.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Clearcell hidradenoma; report of three cases with widespread metastases. Cancer. 1954;7:934-52.
- [Google Scholar]
- Nodular hidradenoma of male breast: Cytohistological correlation. J Cytol. 2011;28:235-7.
- [Google Scholar]
- Atypical and malignant hidradenomas: A histological and immunohistochemical study. Mod Pathol. 2009;22:600-10.
- [Google Scholar]
- Nodular hidradenocarcinoma on the scalp of a young woman: Case report and review of literature. Dermatol Surg. 2004;30:1265-8.
- [Google Scholar]
- Malignant nodular hidradenoma of the skin: Report of seven cases. J Eur Acad Dermatol Venereol. 2008;22:549-54.
- [Google Scholar]
- Malignant nodular hidradenoma of the eyelid: A rare sweat gland tumor. Middle East Afr J Ophthalmol. 2010;17:374-6.
- [Google Scholar]
- Malignant nodular hidradenoma on the scalp: Report of a case with fine needle aspiration cytology features and histologic correlation. Acta Cytol. 2009;53:576-80.
- [Google Scholar]
- Hidradenocarcinoma: A histological and immunohistochemical study. J Cutan Pathol. 2006;33:726-30.
- [Google Scholar]
- Clear cell eccrine carcinomas of the skin. A clinicopathologic study of nine patients. Cancer. 1994;73:1631-43.
- [Google Scholar]






