Translate this page into:
Brainstem hemangioblastoma and sellar mass
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Hemangioblastomas (Hbs) comprise 1.5–2.5% of all intracranial tumors and 7–12% of all posterior fossa tumors.[1] However, sellar Hb is extremely rare.
A 28-year-old lady presented with headache, vomiting of six months’ duration, without any contributory family history. Her physical and neurological examination was normal. Magnetic resonance imaging (MRI) of the brain revealed a solid cystic tumor in the dorsal medulla. The solid component was T1-isointense, T2-heterointense, enhancing brightly on contrast without enhancement of the cyst wall. Radiological possibility of Hb was considered. A sellar lesion measuring 0.72×0.54×0.8 cm was noted, which was T1-hyper and T2-isointense with central hypointense areas, suggestive of tumor with bleed [Figure 1]. The lesion was posterior to stalk without optic chiasm compression. Her haematological, hormonal, and ophthalmological workup and urine catecholamines and metanephrines were normal. Computed tomography (CT) of the abdomen-pelvis did not reveal other systemic tumors associated with von Hippel-Lindau (VHL) disease. She underwent midline suboccipital craniectomy. After durotomy, the tonsils were retracted laterally and the tumor was visualized. The reddish tumor had multiple ventral vascular feeders and was adherent to the floor of the fourth ventricle. The plane of cleavage between the tumor and medulla was explored, the tumor was rapidly devascularized, and en bloc excision was achieved. Histology revealed Hb. She developed lower cranial nerve paresis and was managed with tracheostomy and nasogastric feeds; by the end of three weeks, she was able to take oral feeds and her tracheostomy was closed. In the present case, the diagnosis of the sellar lesion could not be ascertained histologically. Radiological possibility of Rathke's cleft cyst/pituitary apoplexy was considered. In view of concomitant brainstem Hb, the possibility of sellar Hb was considered, though imaging was not characteristic. The asymptomatic sellar lesion was managed conservatively.[2]
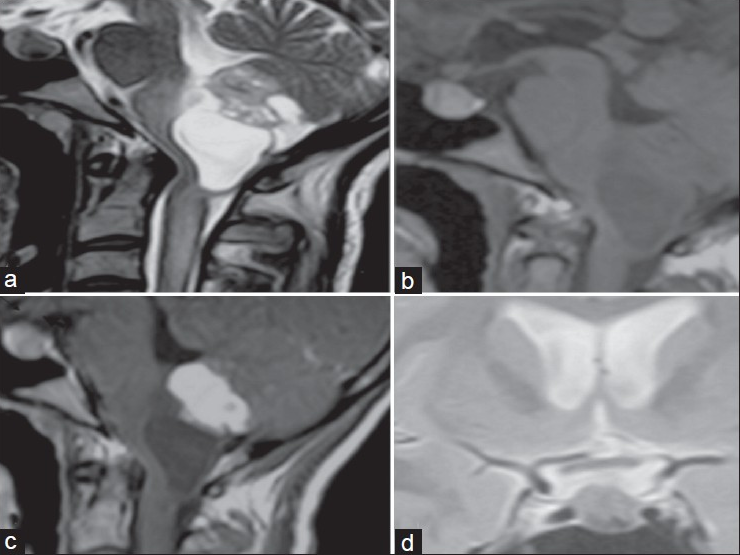
- (a) T2-weighted sagittal image shows heterointense solid cystic tumor in the floor of the fourth ventricle with iso-hypointense lesion in the sellar region. (b) On T1-weighted image, the fourth ventricular lesion is heterointense and the sellar lesion is predominantly hyperintense. (c) Contrast enhanced T1-weighted image shows bright enhancement of the solid component of the fourth ventricular tumor along with the sellar tumor. Optic chiasm (thin arrow) and pituitary stalk (thick arrow) are seen separately. (d) No compression of chiasma by the sellar tumor on T2 coronal
Hbs occur sporadically (66–80%) or with VHL (20–33%).[2] Supratentorial Hb is rare (4–11%),[2] amongst which intraparenchymal are 86.8%, intraventricular are 6.6%, and meningeal are 5.6%.[3] The incidence of sellar Hb varies from <1–29% amongst the supratentorial Hbs.[3] Supratentorial and spinal tumors are usually completely solid compared to the cystic solid variety in the cerebellum. Solid tumors comprise 20–29% and are associated with poorer outcome and higher recurrence compared to cystic tumors.
Usually, sellar-suprasellar Hbs are solid with T1-isointense, T2-hyperintense signals, bright contrast enhancement, and flow voids.[2] Flow voids are a constant feature of Hbs and their evaluation avoids catastrophic bleeding during trans-sphenoidal surgery.[4] VHL with enhancing sellar-suprasellar mass is also suggestive of Hb. Sellar-suprasellar Hb mimics meningioma but accompanying flow voids and absence of dural tail favor Hb. Sellar-suprasellar Hbs arise from the pituitary stalk or optic chiasm.[5] Postoperative panhypopituitarism can occur in sellar-suprasellar Hb.[5] Large Hbs may cause fatal postoperative or subarachnoid hemorrhage but lesions <1.5 cm have an insignificant risk of hemorrhage. In the present case, the sellar lesion was not operated as the patient was asymptomatic.
Radical en bloc excision of tumors is ideal. Dissection should be limited to the gliotic plane between the tumor and the brain, or it may lead to severe bleeding. Excision of the cyst may be useful to enter a deeper location or to release the trapped fourth ventricle. Pituitary stalk Hbs are often asymptomatic and are managed conservatively [Figure 2]. Surgery is offered if the imaging reveals a compression of the surrounding structures.[2] Embolization is useful in large tumors or tumors in difficult locations like the ventral brainstem. Good tumor control and reduced rates of surgery with infratentorial craniospinal irradiation regimen are reported.[6] In a review of 150 patients undergoing radiosurgery, the rate of tumor control was 71% at five years.[5] Radiosurgery is an option for poor surgical candidates. VHL gene loss causes overexpression of the vascular endothelial growth factor, platelet-derived growth factor, and erythropoietin. Treatment includes antiangiogenic agents like interferon (IFN)-α 2a, IFN-α 2b, SU 5416 (Semaxanib), and thalidomide.[7] Thalidomide is cheaper and has been used safely for three years with stabilization of disease. It can be used in surgically inaccessible tumors or patients with multiple Hbs or with significant comorbidities.

- Proposed management algorithm of sellar mass with a proven hemangioblastoma elsewhere
The possibility of Hb should be considered, even in uncommon locations, if a patient has Hb elsewhere. Asymptomatic sellar Hb can be managed conservatively.
References
- Hemangioblastomas of the central nervous system.A 10-year study with special reference to von Hippel-Lindau syndrome. J Neurosurg. 1989;70:24-30.
- [Google Scholar]
- Pituitary stalk hemangioblastomas in von Hippel-Lindau disease. J Neurosurg. 2009;110:350-3.
- [Google Scholar]
- Supratentorial haemangioblastoma not associated with Von Hippel Lindau complex or polycythaemia: Case report and literature review. Br J Neurosurg. 1995;9:81-4.
- [Google Scholar]
- Intrasellar hemangioblastoma: Characteristic prominent vessels on MR imaging. AJR Am J Roentgenol. 2003;180:1480-1.
- [Google Scholar]
- Pituitary stalk hemangioblastoma: The fourth case report and review of the literature. Clin Neurol Neurosurg. 2007;109:292-8.
- [Google Scholar]
- Infratentorial craniospinal irradiation for von Hippel-Lindau: A retrospective study supporting a new treatment for patients with CNS hemangioblastomas. Neuro Oncol. 2011;13:1030-6.
- [Google Scholar]
- Stabilization of a progressive hemangioblastoma under treatment with thalidomide. J Neurooncol. 2004;66:295-9.
- [Google Scholar]





