Translate this page into:
Tuberculum sellae meningiomas: A series of 41 cases; surgical and ophthalmological outcomes with proposal of a new prognostic scoring system
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Context:
Tuberculum sellae meningiomas have proved difficult to treat, partly because of their intimate association with the optic nerves and chiasma, hypothalamus, and internal carotid arteries.
Aims:
The aim of this study is to analyze the degree of influence of various prognostic factors affecting visual outcome; the pattern of visual recovery and develop a scoring system for prognostication.
Settings and Design:
This is a retrospective study carried out from January 2004 till June 2011.
Materials and Methods:
Patients were analyzed on the basis of clinical, radiological, and surgical factors that appeared to affect the outcome. A special scoring system (according to the guidelines of the German Ophthalmological Society) was adopted to quantify the extent of ophthalmological disturbances.
Statistical Analysis Used:
Comparison of categorical variables between the two was performed using chi-square test and a P value of ≤ 0.05 was considered significant. Logistic regression was used when multivariate analysis was required.
Results:
Vision improved in 27% and deteriorated in 7.3%. A prognostic scoring system (score 4–13) was developed depending on the degree of influence of significant prognostic factors. The patients with a score of ≤6 had improved vision postoperatively (44%), whereas none of those with a score > 6 improved. Completeness of visual recovery was perceived in 100% of patients within 3 months. Complete resectability was achieved in 73% of patients.
Conclusions:
The proposed scoring system is very useful in prognosticating the visual outcome of these patients. The patients with a score of ≤6 have the best visual outcome postoperatively. Complete resectability is better achieved with extended bifrontal and unilateral frontal approaches. Short-term postoperative visual outcome is a strong indicator of permanent visual outcome after surgery.
Keywords
Meningioma
prognostic factors
tuberculum sellae
visual outcome
Introduction
Meningiomas of the Tuberculum sellae arise from the limbus sphenoidale, chiasmatic sulcus, and tuberculum. They comprise approximately 3%–10% of all intracranial meningiomas.[1] Correct diagnosis and management require an appreciation of the unique clinical, neuroimaging, and surgery-related features that distinguish these meningiomas from others of the anterior skull base. Tuberculum sellae meningiomas characteristically lie in a suprasellar subchiasmal midline position, displacing the optic chiasm posteriorly and slightly superiorly, and the optic nerves laterally.[1] Slowly progressing visual deterioration is the most common initial complaint, and prompt treatment is directed at preserving and improving vision. Management ideally consists of gross-total resection without injury to neighboring vital structure. The difficulty in surgically excising a Tuberculum sellae meningioma stems from its relationship to the optic nerves and chiasma and to the anterior cerebral and internal carotid arteries and their perforators, which are frequently encased and/or displaced.
The visual outcome of Tuberculum sellae or diaphragm sellae meningiomas is a matter of concern in surgical treatment. Because the inferior surface of the optic nerve and chiasm receives its blood supply from superior hypophyseal arteries that arise from the supraclinoid segment of the internal carotid artery, surgical dissection of the tumor from the inferior side of the optic apparatus is a more formidable procedure than removal of tumors above the optic apparatus.[2]
Materials and Methods
A total of 41 cases of Tuberculum sellae meningiomas underwent surgery by the senior authors (having an experience of more than 10 years) during the period from January 2004 till June 2011. All patients underwent evaluation by CT scanning and MR imaging, with intravenous administration of a contrast agent. The radiological parameters included tumor size, brain–tumor interface, peritumoral edema, arterial encasement, optic canal extension, hyperostosis, etc. These parameters were studied to find their influence on visual outcome. The size of the tumors was calculated on the basis of measurements obtained from MRI images.
Ophthalmological examination and evaluation
Ophthalmological examination included visual acuity, fundoscopy, and visual field charting. The findings were analyzed according to the guidelines of the German Ophthalmological Society.[3] Tables [Figure 1] were established to assess visual acuity and visual field defects, for which any combination in both eyes was assigned special score. The scores for visual acuity and visual field defects in each patient were added, thus providing the visual impairment score (VIS), which enabled an exact comparison between different examinations in each patient. The VIS ranges from 0 to 100.
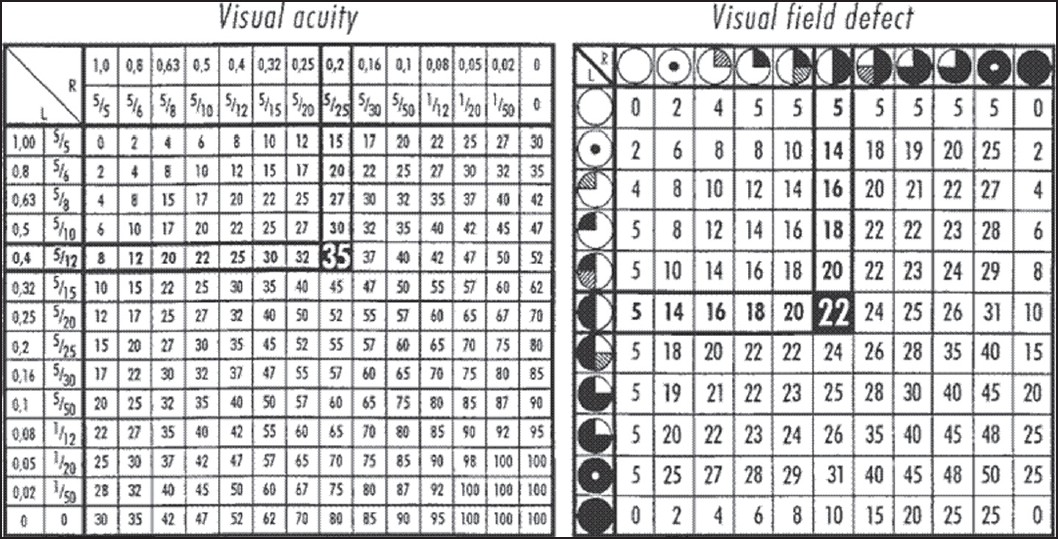
- Tables demonstrating visual acuity and visual field deficit according to the German Ophthalmological Society for calculation of the visual impairment score. For example, a patient with visual acuity of 0.4 (4/10) in the left eye and 0.2 (2/10) in the right eye has a visual acuity impairment score of 35, and if bitemporal visual field deficit is associated, a visual field impairment score of 22. The addition of both the scores gives the visual impairment score (VIS). The score ranges from 0 to 100
Surgical procedure
The surgical approaches included extended bifrontal, unilateral frontal (lateral supra orbital and anterior interhemispheric), pterional, and fronto-temporo-orbito-zygomatic (FTOZ). Optic canal decompression was performed when there was tumor extension [Figure 2] into it. The extent of tumor resection was based on Simpson grading. The surgical approaches were tailored as to surgeons’ choice and experience.

- Pre-op (1a) and post-op (1b) images of Tuberculum sellae meningioma with left optic canal extension.
Follow-up examination
Follow-up examinations were scheduled at 1, 3, and 6 months postoperatively and at irregular intervals thereafter, to assess ophthalmological function and imaging studies. The follow-up ranged between 6 months and 4 years.
Statistical analysis
Data were entered in Excel software (Microsoft, Seattle, WA, 2002) and was analyzed using SPSS software, version 15.0 (SPSS, Inc., Chicago, IL). Means and standard deviations were computed for continuous variables and marginal distributions for categorical variables. Comparison of categorical variables between the two was performed using chi-square test and a P value of ≤ 0.05 was considered significant. Logistic regression was used when multivariate analysis was required.
Results
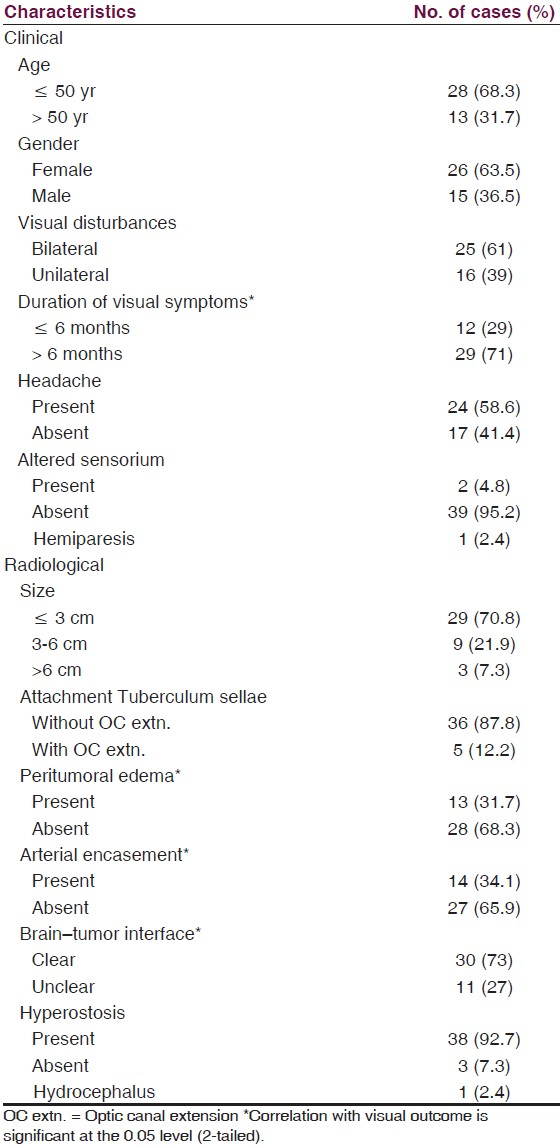
There were 26 women and 15 men with a female to male ratio of 1.7:1. The patient characteristics and radiological parameters are as shown in Table 1.
Ophthalmological outcomes [Table 2]
The overall visual preservation (improvement + stable vision) was 92.7%. Visual deterioration occurred in 7.3% (3 of 41 patients) and vision improved in 27% (11 of 41 patients). Completeness of visual recovery was perceived in 100% of patients within 3 months.
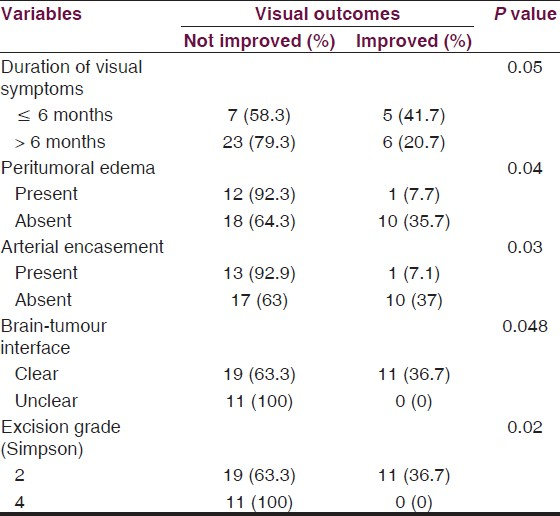
Patient characteristics-There was a clear correlation between the duration of symptoms and visual prognosis. The best prognosis was found in patients in whom the duration of symptoms was less than 6 months than in those patients with duration more than 6 months. This finding was statistically significant (P ≤ 0.05). None of the other clinical characteristics, including, pre-op VIS, age, gender, etc., had significant influence on visual outcome.
Radiological parameters-Visual outcome had significant correlations with peritumoral edema (P ≤ 0.04), arterial encasement by the tumor (P ≤ 0.03), and brain-tumor interface (P ≤ 0.048). The other parameters, tumor size, optic canal extension, hyperostosis, etc., did not have significant influence on the visual outcome.
Surgical excision - 30 out of 41 patients (73%) had Simpson grade 2 excisions, while the rest underwent Simpson grade 4 excisions owing to tumor rests around the vessels or the optic nerve. There was a significant correlation between excision grade and visual outcomes with better outcomes when total excision is performed (P ≤ 0.02).
Complications
Postoperatively, the complications observed were cerebrospinal fluid (CSF) rhinorrhoea in 2 patients, transient diabetes insipidus in 1 patient, operative site hematoma in 1 patient, and contralateral paraesthesias in 1 patient (caudate infarct). All these patients were managed conservatively. Two patients of CSF rhinorrhoea developed meningitis and died. Both these patients had accidental sphenoid sinus opening during drilling of Tuberculum sellae with the unilateral subfrontal approach, which was subsequently packed with fat. The overall rate of nonvisual morbidity was 12.2% (5 of 41 patients) and mortality was 4.9%.
Pattern of recovery
In this study, the overall visual improvement was 27% (11 patients). Out of these, 5 patients (45.5%) showed visual improvement within the first month of follow-up, 6 patients (54.5%) within 3 months of surgery. Completeness of visual recovery was perceived in 100% of patients within 3 months. The VIS at 3 months and 6 months follow-up showed significant correlation (P ≤ 0.032 and 0.023, respectively) with visual outcome.
Factors affecting complete resectability [Table 3]
Various surgical factors were analyzed to study their correlation with resectability. The significant factors which influenced complete resectability include tumor size < 6 cm, peritumoral edema, arterial encasement, and surgical approaches-extended bifrontal and unilateral frontal. 84% of cases operated by extended bifrontal and unilateral frontal approaches had complete resection compared to only 40% of those operated by other approaches (pterional and FTOZ). Other factors like brain-tumor interface, hyperostosis, optic canal extension, etc., did not have any significant influence on complete resection of the tumor.

Prognostic scoring system
Various clinical and radiological factors affecting visual outcome were studied and their significance analyzed using SPSS software, version 15.0 (SPSS, Inc., Chicago, IL). Based on their significance, we developed a scoring system to prognosticate the patients. A logistic regression model (multivariate analysis) was used to evaluate the degree of influence of these confounding factors on visual outcome. In this study, duration of visual symptoms, peritumoral edema, arterial encasement, brain-tumor interface, and grade of excision are the five significant factors affecting the visual outcome. Total score which could be allotted for the particular variable depending on the degree of influence on visual outcome was calculated using this model in the SPSS software. Based on these scores (adjusted to the nearest whole number), individual scores were assigned among the significant variables. Combined score of all the significant factors was calculated with a maximum of 13 and minimum score of 4 [Table 4]. The patients who had a total score of ≤ 6 had better visual outcomes (44% improvement, 11 out of 25 patients). None of the patients with a score of >6 (0 out of 16 patients) showed any visual improvement [Table 5].


Discussion
The primary symptom leading to the diagnosis of a Tuberculum sellae meningioma is visual compromise. Therefore, the aim of surgery is to achieve improvement of vision. During the macrosurgical era, improvement varied between 40% and 63%.[4–6] The range of improvement rates in microsurgical series is 25%–80% 3.[7–12] In previous reports, visual acuity improved in 28% to 80% of patients, remained unchanged in 9% to 64%, and deteriorated in 7% to 33%.[3–581013–19] In our series, vision improved in 27% of the patients, remained unchanged in 65.7%, and deteriorated in 7.3%. The key to preserving visual function is to minimize direct manipulation or trauma to the optic nerves and avoid injury to the blood supply of the optic apparatus.[310]
Several studies have described various factors affecting visual outcome. Fahlbusch and Schott[3] mentioned that young age and short symptom duration are good prognostic factors for visual outcome. Goel et al.[8] proposed symptom duration in addition to preoperative visual function, anterior cerebral artery encasement, and tumor size as valuable prognostic factors. Zevgaridis et al.[20] suggested prognostic factors such as age, symptom duration, preoperative visual function, and arachnoid membrane intactness. Margalit et al.[1121] emphasized the importance of optic nerve encasement, tumor size, and preoperative visual function. Park et al.[22] described preoperative visual function and symptom duration as the prognostic factors affecting outcomes.
There has not been any study till date which can prognosticate visual outcome in patients based on a scoring system. Goel et al.[8] in their study have proposed a scoring system based on clinical and radiological factors to predict the complete resectability of these tumors. The degree of influence of each prognostic factor is different and affects visual outcome in a unique way. We in our study have developed a scoring system which is easy to use and is very useful in predicting the visual outcome for these patients. This system takes into account the unique influence of these factors. As seen in the Table 5, the patients with a score of >6 did not have any visual improvement in the post-op period, which is highly significant (P = 0.006). The various significant variables and the possible reasons with the review of literature are as discussed below.
-
Duration of visual symptoms-Zevgaridis et al.[20] in their study found that patients with symptoms for more than 7 months showed unfavorable outcome. In the series by Fahlbusch and Scott,[3] there was a clear correlation between the duration of symptoms and the visual prognosis. Pamir et al.,[23] Li et al.,[24] Bassiouni et al.,[25] Andrews and Wilson,[14] etc., have reported similar effect of symptom duration on outcomes. The possible reason is that compressive mechanical injury leads to small vessel compromise and demyelination, especially in patients with a long duration of visual loss before surgery.
-
Peritumoral edema-In the study by Pamir et al.[23] peritumoral edema had a significantly worse visual outcome. We in our study too found similar results. Peritumoral edema in meningiomas is correlated with a higher proliferative index and a higher angiogenetic activity.[23] Therefore, it may be speculated that the dismal visual outcome might be related to a higher growth rate and accelerated course of nerve compression.
-
Brain–tumor interface-In the cohort series by Pamir et al.,[23] an intact arachnoid plane could be identified in 83.33% of the patients and the visual outcome in patients with an intact arachnoid plane was significantly better than those, in whom no plane could be identified. The importance of this plane in achieving a safe resection has been stressed by several authors. Zevgaridis et al.[20] were the first group to show that its presence was also correlated with a better visual outcome. Early identification and preservation of the arachnoid anatomy facilitates easy dissection of the tumor from its surroundings and optic and chiasmatic vessels.
-
Arterial encasement-The arterial encasement by the tumor has a direct bearing on the complete resectability of the tumor, which in turn has significant influence on the visual outcome, as seen in our study.
-
Grade of excision-Several studies have stressed that complete resection should not be attempted at the cost of visual deterioration or hypothalamic dysfunction. The percentage of gross total tumor resection of these meningiomas ranged from 35% to 100%.[3–1013141819] The extent of tumor resection has improved with the advent of modern microsurgery, with a total resection rate of 35 to 76% in macrosurgical series, compared with 58% to 100% in microsurgical series.[26] Zevgaridis et al.[20] found no significance with the extent of tumor removal. The results by Pamir et al.[23] indicate that contrary to the aforementioned hypothesis the visual outcome was better in totally resected tumors. An intact arachnoid plane has been reported in most tumors except for very large ones and this plane allows for aggressive tumor removal with preservation of surrounding structures.
There are contradictory reports on the effect of tumor size on visual outcome. Several authors have shown that larger tumors had worse outcome while others have disputed this notion. Rosenstein and Symon[18] found tumors smaller than 3 cm to be associated with better visual outcomes than tumors larger than 3 cm in diameter. Andrews and Wilson,[14] Schick and Hassler,[27] Raco et al.,[28] Li et al.,[24] etc., too have found similar influence of tumor size on visual outcome. Other authors like Fahlbusch and Scott,[3] Ehlers and Malmros,[3] Pamir et al.,[23] Bassiouni et al.,[25] and Zevgaridis et al.,[20] etc., did not find any effect of tumor size on visual outcomes. We in this study too found similar results. The degree of visual impairment due to the impingement on the optic nerves and chiasm are quite variable. Even large meningiomas may be asymptomatic for prolonged periods despite significant compression of the optic apparatus.[25]
There are various transcranial approaches used to resect these meningiomas: (1) bicoronal subfrontal, (2) unilateral subfrontal, (3) pterional transsylvian, (4) anterior interhemispheric, (5) extended bifrontal, (6) skull base technique, and (7) FTOZ. The incidence of the preservation of the olfactory nerves is theoretically better with the use of anterior interhemispheric approach for Tuberculum sellae meningiomas. The rate of total excision is probably better with the use of an anterior interhemispheric approach, because the best exposure of the ACA complex can be achieved using this approach.[29] Initial debulking of the tumor should start from within the center, where no vital structures are present. The arachnoid plane is then delineated, starting at the contralateral optic nerve and working to the undersurface of the optic chiasm and then along the ipsilateral nerve. With this technique, the surgeon remains within the subdural space as much as possible, minimizing direct injury to the optic nerves.[30] During resection of the meningioma, small vessels observed in the stretched arachnoid layer should not be coagulated. By preserving these vessels, there is a better chance for visual function improvement.
The extended frontal and unilateral frontal approaches (lateral supra orbital[31] and anterior interhemispheric) had better rates of complete resection (84%) compared to other approaches (40%) in our study. The extended bifrontal craniotomy is favored for approaching large Tuberculum meningiomas more than 3 cm.[32] This exposure not only allows for wide exposure and flexibility of surgical trajectories, but also limits excessive retraction during these long operations. The advantage is that it provides a wide and direct view of both optic nerves, as well as internal carotid and anterior cerebral arteries.
Various authors have considered the size of the tumor to be a crucial factor that indicates possible surgical difficulties. Large tumors cause more severe stretching of the adjoining nerves and vessels, and consequently, the resection is more difficult. As the tumor grows in size, the edema surrounding it increases and the tumor encases the nearby arteries. The intactness of the arachnoid plane is lost with large tumors thus, causing difficulty in complete resection with preservation of surrounding structures.
Goel et al.[8] have described a scoring system based on clinical and radiological parameters to predict resectability of these tumors. In their study, they have found significant influence of arterial encasement on the grade of excision. We too found similar results in our study. There is no clear arachnoid plane between the encased artery and the tumor, thus affecting the complete resectability. In case of engulfment by tumor lobulations, the arachnoid plane is maintained making the dissections simpler and complete resection can be achieved.
One aspect, hardly investigated in previous literature, is the ability and duration of visual recovery. Gregorius et al.[4] described that 25% of their patients experienced visual improvement within the first day, 25% within the first 10 days, 25% within the first 2 months, and 1/16 within the first year after operation. Rosenstein and Symon[18] reported a rapid improvement within the first two weeks in 62/63 patients. However, completeness of visual recovery was perceived in 37/63 of their patients within the first 2 months, in 14/63 within the first 6 months, and in 11/63 within the first year. Puchner et al.[17] suggested that visual recovery is not completed within the first 10 days after surgery in at least 50% of patients. Park et al.[22] had concluded that short-term postoperative visual outcome was a strong indicator of permanent visual outcome after surgery. Completeness of visual recovery was perceived in 100% of patients within 3 months in our study, and VIS at follow-up had significant correlation with visual outcome. The optic nerve, although it is known as an extremely sensitive part of the brain, bears a certain ability to recover over longer time intervals.[17] However, renewed visual deterioration after some initial recovery is highly indicative of tumor recurrence.[22]
Conclusions
In our series of Tuberculum sellae meningiomas, it was possible to achieve a favorable outcome with a low incidence of morbidity and mortality. The patients with a score of ≤ 6 (proposed scoring system) have best visual outcome postoperatively. Short-term postoperative visual outcome is a strong indicator of permanent visual outcome after surgery. Complete resectability of these meningiomas is better achieved with extended bifrontal and unilateral frontal approaches. Meticulous care should be taken to repair the accidental sphenoid sinus opening during unilateral frontal approaches, in order to reduce the post-op morbidity and mortality.
Source of Support: Nil
Conflict of Interest: None declared
References
- The microsurgical nuances of resecting tuberculum sellae meningiomas. Neurosurgery. 2005;56:411-7.
- [Google Scholar]
- Pterional surgery of meningiomas of the tuberculum sellae and planum sphenoidale: Surgical results with special consideration of ophthalmological and endocrinological outcomes. J Neurosurg. 2002;96:235-43.
- [Google Scholar]
- Loss and recovery of vision with suprasellar meningiomas. J Neurosurg. 1975;42:69-75.
- [Google Scholar]
- Importance of failing vision in early diagnosis of suprasellar meningiomas. Br Med J. 1962;2:5-7.
- [Google Scholar]
- Surgical management of suprasellar meningioma. Part 1: The influence of tumor size, duration of symptoms, and microsurgery on surgical outcome in 101 consecutive cases. J Neurosurg. 1984;61:633-41.
- [Google Scholar]
- Tuberculum sellae meningiomas 1991
- Tuberculum sellae meningioma: A report on management on the basis of a surgical experience with 70 patients. Neurosurgery. 2002;51:1358-63.
- [Google Scholar]
- Microsurgical management of tuberculum sellae meningiomas. Results in 28 consecutive cases. Surg Neurol. 1986;26:37-44.
- [Google Scholar]
- Tuberculum sellae meningiomas: Microsurgical anatomy and surgical technique. Neurosurgery. 2002;51:1432-9.
- [Google Scholar]
- Tuberculum and diaphragma sella meningioma--surgical technique and visual outcome in a series of 20 cases operated over a 2.5-year period. Acta Neurochir (Wien). 2007;149:1199-204.
- [Google Scholar]
- Meningiomas of the anterior cranial fossa floor. Review of 67 cases. Acta Neurochir (Wien). 1994;129:26-30.
- [Google Scholar]
- Suprasellar meningiomas: The effect of tumor location on postoperative visual outcome. J Neurosurg. 1988;69:523-8.
- [Google Scholar]
- Visual outcome of tuberculum sellae meningiomas after extradural optic nerve decompression. Neurosurgery. 2006;59:570-6.
- [Google Scholar]
- Treatment of tuberculum sellae meningiomas: A long-term follow-up study. J Clin Neurosci. 2001;8(Suppl 1):26-31.
- [Google Scholar]
- Suprasellar meningiomas--neurological and visual outcome at long-term follow-up in a homogeneous series of patients treated microsurgically. Acta Neurochir (Wien). 1998;140:1231-8.
- [Google Scholar]
- Surgical management of suprasellar meningioma. Part 2: Prognosis for visual function following craniotomy. J Neurosurg. 1984;61:642-8.
- [Google Scholar]
- Suprasellar and olfactory meningiomas. Report on a series of 153 personal cases. Acta Neurochir (Wien). 1983;67:181-94.
- [Google Scholar]
- Meningiomas of the sellar region presenting with visual impairment: Impact of various prognostic factors on surgical outcome in 62 patients. Acta Neurochir (Wien). 2001;143:471-6.
- [Google Scholar]
- Meningiomas involving the optic nerve: Technical aspects and outcomes for a series of 50 patients. Neurosurgery. 2003;53:523-32.
- [Google Scholar]
- Surgically treated tuberculum sellae and diaphragm sellae meningiomas: The importance of short-term visual outcome. Neurosurgery. 2006;59:238-43.
- [Google Scholar]
- Outcome determinants of pterional surgery for tuberculum sellae meningiomas. Acta Neurochir (Wien). 2005;147:1121-30.
- [Google Scholar]
- Surgical management of tuberculum sellae meningiomas. J Clin Neurosci. 2007;14:1150-4.
- [Google Scholar]
- Tuberculum sellae meningiomas: Functional outcome in a consecutive series treated microsurgically. Surg Neurol. 2006;66:37-44.
- [Google Scholar]
- Tuberculum sellae meningiomas: Clinical outcome considering different surgical approaches. Neurosurgery. 2006;59:1019-28.
- [Google Scholar]
- Surgical management of tuberculum sellae meningiomas: Involvement of the optic canal and visual outcome. J Neurol Neurosurg Psychiatry. 2005;76:977-83.
- [Google Scholar]
- Meningiomas of the tuberculum sellae.Our experience in 69 cases surgically treated between 1973 and 1993. J Neurosurg Sci. 1999;43:253-60.
- [Google Scholar]
- Lateral supraorbital approach applied to anterior clinoidal meningiomas: Experience with 73 consecutive patients. Neurosurgery. 2011;68:1632-47.
- [Google Scholar]
- Extended bifrontal craniotomy for midline anterior fossa meningiomas: Minimization of retraction-related edema and surgical outcomes. Neurosurgery. 2006;59:ONS 426-33.
- [Google Scholar]






