Translate this page into:
Decompressive craniectomy in patients with cerebral infarction due to malignant vasospasm after aneurysmal subarachnoid hemorrhage
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Aim:
The authors present their experience and the clinical results in decompressive craniectomy (DC) in patients with vasospasm after aneurysmal subarachnoid hemorrhage (SAH).
Materials and Methods:
Between 2002 and 2010, six patients underwent DC due to cerebral infarct and edema secondary to vasospasm after aneurysmal SAH. Four patients were male, and two were female. The age of patients ranged between 33 and 60 (mean: 47,6 ± 11,4). The follow up period ranged between 12 to 104 months (mean: 47,6 ± 36,6). The SAH grading according World Federation of Neurosurgeons (WFNS) score ranged between 3 to 5.
Results:
Last documented modified Rankin Score (mRS) ranged between 2 to 6. One patient died in the following year after decompression due to pneumonia and sepsis. Two patients had moderate disability (mRS of 4) and three patients continue their life with minimal deficit and no major dependency (mRS score 2 and 3).
Conclusion:
DC can be a life-saving procedure which provides a better outcome in patients with cerebral infarction secondary to vasospasm and SAH. However, the small number of the patients in this study is the main limitation of the accuracy of the results, and more studies with larger numbers are required to evaluate the efficiency of DC in this group of patients.
Keywords
Cerebral infarction
decompressive craniectomy
subarachnoid hemorrhage
vasospasm
Introduction
Aneurysmal subarachnoid hemorrhage (SAH) is a devastating pathology due to its primary and secondary injurious impact on the brain, with mortality rates up to 70%.[1] It is accepted now that the best treatment of aneurysmal SAH is early surgical intervention, either by microsurgical or endovascular methods, to prevent re-bleeding and for safer management of vasospasm after secured aneurysm. In some patients, severe malignant vasospasm may develop and becomes resistant to conventional medical and endovascular treatments. This result is wide spread infarction and edema with secondary increased intracranial pressure (ICP) and decreased brain blood perfusion. Decompressive craniectomy (DC) is a well explained technique in patients with malignant middle cerebral artery (MCA) infarctions, and it is used also as prophylactic manners in patients with SAH and hematoma at the admission.[2–5] Only few studies described the effect of DC performed in patients with ischemic events secondary to malignant vasospasm after aneurysmal SAH.[6–8]
In this article, the authors present their series of DC in patients with aneurysmal SAH who developed treatment-resistant malignant vasospasm and cerebral infarction.
Materials and Methods
Between 2002 and 2010, 306 patients with anterior circulation aneurysm were operated by microsurgical methods. Of these patients, 49 patients (16.2%) presented with SAH. Standard treatment for ruptured aneurysms in our clinic is clipping the neck of the aneurysm in the first 24 hours. Intraventricular cerebrospinal fluid (CSF) drainage catheter was routinely inserted in cases with World Federation of Neurosurgeons (WFNS) score of 3 or more, and in some cases with WFNS score of 2 or less according to the surgeon's personal decision about the possibility of complication risk. In the post-operative period, especially after day 7, patients developing new neurologic (decreased consciousness, hemiparesis, aphasia) or suspicious systemic signs (headache, unexplained subfebrile fever) were immediately scanned with brain CT. Cases with radiologic ischemic signs corresponding to vascular territory were accepted with the diagnosis of vasospasm. Cases with normal radiologic findings without gross ischemic changes immediately underwent digital subtraction angiography (DSA) to confirm the diagnosis. With this approach, diagnosis of vasospasm was made early by the correlation of the clinical and radiological findings, and before deterioration of the patient due to late complications of edema and herniation. Treatment of vasospasm was started immediately once the diagnosis is made (generally within 1-4 hours from the clinical onset) with standard 3-H treatment, which includes Hypertension to maintain the systolic blood pressure (SBP) between 200-220 mmHg, Hypervolemia with crystalloid and/or colloid fluids to maintain the central venous pressure (CVP) between 10-12 mmHg, and Hemodilution to maintain the hematocrit between 30-35%. Nipodepine was not used in our patients and it was not included in the standard treatment of vasospasm. The endpoint of 3-H therapy was the neurological improvement. Cases not responding to 3-H therapy were managed with balloon angioplasty in a time period of 6 – 12 hours from the onset of the symptoms. All cases in our series with medical treatment- resistant vasospasm were with significant proximal artery occlusion, and balloon angioplasty was used as the main endovascular treatment method for these cases. Intra-arterial Papaverin injection was performed after dilation of the proximal arteries with balloon angioplasty to effect distal branches. However, when balloon angioplasty was not successful for proximal arteries, chemical angioplasty was not performed. Indications of the surgical intervention were: 1) clinically and radiologically approved vasospasm, 2) failure of medical and endovascular treatment of vasospasm, 3) resistant increase of intracranial pressure (ICP) >20 mmHg due to developed ischemia and edema [Figure 1]. This group included 6 patients, 4 with middle cerebral artery (MCA) bifurcation and 2 with anterior communicating artery (AComA) aneurysms. Four of these patients were male (66.6%), 2 were female (33.3%). The age of patients ranged between 33 and 60 (mean: 47.6±11.4). The Glasgow Coma Scale (GCS) at the time of admission ranged between 6 and 13, and the SAH grading WFNS score ranged between 3 to 5. Intraventricular CSF drainage catheter was inserted all patients in this series. Early GCS after aneurysm clipping (post-operative day 2) showed improvement and ranged between 8 – 15. Onset of confirmed clinical vasospasm ranged between 5- 10 day after SAH. All patients were with medical and endovascular treatment resistant vasospasm. Period between the diagnosis of vasospasm and DC ranged between 2-3 days. GCS just before decompression varied between 5 and 10. Follow up period changed between 12 to 104 months (mean: 47.6 ± 36.6). During the follow-up period, patients are examined in day 10 and at months 2, 6, 9,12 and at the time of this study. The dependency of the patients were evaluated with modified Rankin Scale (mRS) (score 1-6), which takes as criteria only dependency instead of neurologic and general condition [Table 1]. Radiologic evaluation was performed when necessary. Data of the patients are summarized in Table 2.
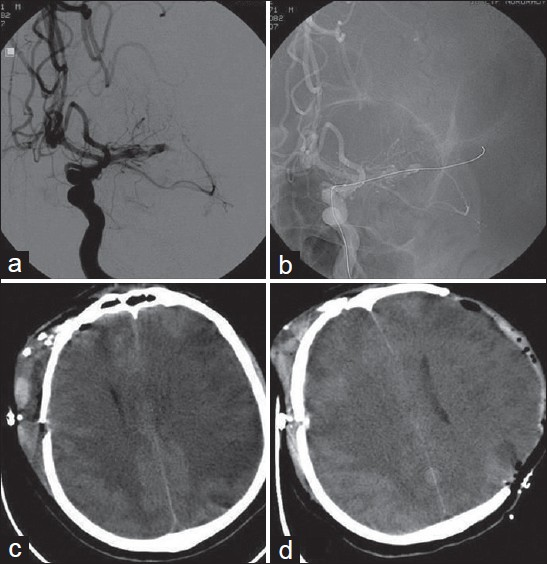
- A case of SAH due to ruptured right MCA bifurcation aneurysm. Malignant vasospasm was developed in the territory of the left MCA (a) Cerebral angiography after surgical clipping of the aneurysm and developement of the vasospasm showing severe vasospasm in the left MCA (b) Cerebral angiographic views during the attempt of angioplasty of the left MCA (c) Brain CT-scan showing ischemic changes and edema in the contralateral MCA territory with midline shift. (Note: right intraventricular catheter is not visible as this CT section is immediately below the tip of the catheter which slightly upward migrated after subfalcian herniation) (d) Brain CT-scan obtained after decompressive craniectomy performed via a left fronto-parietotemporal craniectomy and removal of the intraventricular catheter


Surgical technique
At the side of the prominent edema and mass effect, a large question mark skin incision including frontal, parietal and temporal bones were made, followed by a minimum of 12 cm fronto-parieto-temporal craniotomy [Figure 2a]. In cases the infarction and edema were at the same side of operated aneurysm, re-incision was made with an extension incision from the dome of the flap toward posterior to the level of the posterior border of the ear, and bone resection was extended obtaining to bone flaps [Figure 2b]. Bone resection reaching the middle temporal fossa is always performed. The dura is opened in “C” fashion and tailored with incisions towards midline. Brain parenchyma resection is never performed. Duraplasty is done using autologous pericranial fascia. Removed bone flaps preserved inside of fatty tissue of abdomen of patients. In our series, cranioplasty was performed after 6 months in 1 patient, after 8 months in 3 patients, after 9 months in 1 patient, after 10 months in 1 patient and after 12 months in 1 patient.

- (a) A schematic drawing demonstarting the skin incision for DC in previously unoperated side (b) and in case with previous operation with pterional craniotomy
Results
DC performed in 6 patients with SAH from MCA bifurcation and AComA aneurysms. In patients with MCA bifurcation aneurysms, the aneurysm was on the left side in two patients and on the right side in the other two patients. The infarction and edema were on the same side in 3 patients with MCA bifurcation aneurysm. On the other hand, the infracted area was in the contralateral side in 1 patient with left MCA bifurcation aneurysm. In two patients with AComA aneurysms, infarct and edema was in the left side in 1 patient and the in right side in the other patient.
There were no intra-operative or post-operative complications related to the surgical procedure. One patient with MCA bifurcation aneurysm died 12 month after DC due to pulmonary infection and sepsis. The mRS score was 5 in 1 patient (16.7%), 4 in 2 patients (33.3%), 3 in 1 patients (16.7%) and 2 in 2 patients (33.3%). We also evaluated patients with mRS score of 3 or below as ‘good outcome’; and those with mRS of 4 or above as ‘bad outcome’. On the basis of these parameters, 50% of our patients had bad outcome, and 50% of patients had good outcome. Also, the outcome of this series demonstrated by mRS scores showed correlation with the GCS scores early post-clipping and before DC. Patients with higher mRS scores had higher GCS scores after clipping and before DC [Table 2].
Discussion
Cerebral stroke, both ischemic and hemorrhagic, is a devastating clinical condition, which is the second leading cause of death worldwide only secondary to coronary diseases.[9] Although the mechanism of damage varies with every different cause of cerebral stroke, such as cerebrovascular infarction, hypertensive intracerebral hemorrhage, SAH and traumatic brain injury, cerebral stroke causes damages due to its primary and secondary injurious impacts.[10–13] The management of cerebral stroke patients rests on the prevention of secondary brain damage. High intracranial pressure within the fixed-volume skull can lead to secondary brain damage, herniation, permanent neurological damage, or even death. Patients with space-occupying hemispheric infarctions have a poor prognosis, with fatality rates of up to 80%.[4] For this reason, new additions to the arsenal of the physicians are always welcomed. DC is a neurosurgical procedure which is defined to treat high intracranial pressure. It includes the removal of calvarial bones to create free space which the brain can occupy under the scalp, aiming to minimize ischemic damage by increasing cerebral blood flow.[101213]
DC after MCA infarction is a well defined technique and is the topic of many actual studies.[2–4] These studies demonstrated the benefits of DC in patients with malignant MCA infarctions. At 12 months, these trials revealed a significant difference between surgical and conservative treatment groups, with 75% and 23% success, respectively, with primary outcome scale measure of mRS 4. However, this score means severe disability and dependency rates of patients. Because of these conclusions, the efficacy of this procedure to provide a better quality of life was questionable in spite of the approved life-saving effect. On the other hand, in cases of outcome measure of mRS of 3, success rates are 43% and 21% in surgical and conservative groups respectively.[11] However, these authors proposed that surgical decompression should be considered in patients up to 60 years old and should be performed within 48 hours of deterioration.[3] Also, DC was used as a prophylactic step during primary operation, in poor grade patients with SAH presenting with large hematomas.[14] Smith et al. performed a modified pterional craniectomy including duraplasty in 8 patients with poor grade SAH due to MCA aneurysms.[1] With all patients ICP decreased below 20 mmHg after procedure. They reported only 1 mortality, while 7 patients had good outcome score. An important issue pointed in our study was that the authors emphasized the early intervention even prior the clinical symptoms arise, based on ICP and radiologic follow-up.
Other than pterional craniectomy, reports of DC was performed as bilateral craniectomy in patients with anterior communicating artery aneurysm and a posterior fossa decompression were described with good outcome.[1415] In a study of Shirmer et al. they showed that even in cases of SAH without large intracranial hematoma, DC led to significant decrease of medically unresponsive ICP elevation.[16] They concluded that DC performed in 48 hours after aneurysmal rupture is associated with better outcome rates.[16] On the other hand, in a case-controlled study of D’Ambrosio et al. for DC after SAH, a non-significant increase was found in short-term survival in 12 patients treated with DC, compared to patients treated conservatively after the initial aneurysm treatment.[17] An overall poor quality of life, measured with functional and emotional tests, was experienced by the surviving members of the DC group. Thus, the authors were skeptical of the benefits of DC.[2] However, a close look to patients’ demographic data in this study showed that younger patients, who were more likely to benefit from DC, underwent the procedure after 72 hours of initial aneurysm treatment. This delay of the treatment might had negative impact upon outcome. On the other hand, 4 of 8 patients who underwent DC before 48 hours, were older than 60 years old, which were not also considered in the group who would most benefit from DC. In general, DC in the category of malignant vasospasm after SAH was found to have a favorable effect on the prognosis, however, it still has the lowest beneficial outcome group when compared to other categories (e.g.: hematoma, stoke, trauma, etc.), ranging from 10-26.6%.[67]
In our study, as the main issue was to decrease ICP when increased to prevent brain herniation, lowering the ICP when it reaches 20 was beneficial and DC was the alternative option after the failure of other means. Studies showed that successful management of increased ICP with less invasive approaches is efficacious and improve outcome after aneurysmal SAH and its related complications, such as edema, infarction and/ or hematoma.[6718] For this reason resistant increased ICP >20 mmHg was considered the main indication for DC after such cases of aneurysmal SAH.[67] As described earlier, all of our cases were operated via a fronto-parieto-temporal craniectomy. We performed bone removal adjacent to the swelling hemisphere. In our experience this amount is enough to obtain eligible increase in clinical condition. DC for MCA territory infarction was already explained in details in different studies.[2–4] One patient with AComA aneurysm had already a very bad neurological status while admission, thus the expected benefit from DC was low. However, the other patient with AComA aneurysm tolerated the surgery well and saw benefit with a good outcome score (mRS:3).
In our series, one patient died after one year of DC due to pulmonary infection, while mRS score was 5 at the time of discharge. In many studies, patients with mRS score of 3 or below were considered with ‘good outcome’.[5] Accordingly, in our study we also evaluated patients with mRS score of 3 or below as ‘good outcome’; and those with mRS of 4 or above as ‘bad outcome’. On the basis of these parameters, 50% of our patients had bad outcome. Patients in this group are those with bad SAH score at admission. On the other hand, 50% of patients had good outcome and which is considered favorable compared with other studies. Outcome scores did not differ between patients who had SAH due to MCA bifurcation or AComA aneurysm. The main drawback in this study is the small number of the patients, which limits the accuracy of the results and more studies with larger numbers are required to evaluate the efficiency of DC in this group of patients. Also, the small number of the patients leads to the inability to make a precise comment upon the impact of patient age on outcome score. All our patients were under 60 years old, thus the expectancy of benefit was high. Also, there was no control group in this study, as we did not find it proper to withhold surgery from patients with resistant increase in ICP. For this reason, we compared the results of this series with findings observed in other similar studies. However, more studies with larger numbers and control groups are needed to obtain more precise results.
Conclusion
Our series showed that DC can be a life-saving procedure which provides a better outcome in patients with cerebral infarction secondary to vasospasm and SAH. DC was most beneficial when it was performed in an immediate fashion after the documentation of the resistant increase of ICP in patients younger than 60 years old. Early intervention may have a great influence on the outcome. In spite of the favorable results in this series, the small number of the patients is the main limitation of the accuracy of the results, and more studies with larger numbers are required to evaluate the efficiency of DC in this group of patients.
Source of Support: Nil
Conflict of Interest: None declared
References
- Outcome and cost of aneurysmal subarachnoid hemorrhage. Neurosurg Clin N Am. 2010;21:235-46.
- [Google Scholar]
- Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery infarction with Life-threatening Edema Trial [HAMLET]): A multicentre, open randomised trial. Lancet Neurol. 2009;8:326-33.
- [Google Scholar]
- Decompressive Surgery for the Treatment of Malignant Infarction of the Middle Cerebral Artery (DESTINY): A randomized, controlled trial. Stroke. 2007;38:2518-25.
- [Google Scholar]
- DECIMAL Investigators: Sequential-design, multicentre, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL Trial) Stroke. 2007;38:2506-17.
- [Google Scholar]
- Proposed use of prophylactic decompressive craniectomy in poor-grade aneurysmal subarachnoid hemorrhage patients presenting with associated large sylvian hematomas. Neurosurgery. 2002;51:117-24.
- [Google Scholar]
- Decompressive hemicraniectomy after aneurysmal subarachnoid hemorrhage. World Neurosurg. 2010;74:465-71.
- [Google Scholar]
- Decompressive craniectomy in aneurysmal subarachnoid hemorrhage: Relation to cerebral perfusion pressure and metabolism. Neurocrit Care. 2009;11:384-94.
- [Google Scholar]
- Mortality by cause for eight regions of the world: Global burden of disease study. Lancet. 1997;349:1269-76.
- [Google Scholar]
- Effect of early bilateral decompressive craniectomy on outcome for severe traumatic brain injury. Turk Neurosurg. 2010;20:382-9.
- [Google Scholar]
- Factors affecting the outcome of decompressive craniectomy for large hemispheric infarctions: A prospective cohort study. Acta Neurochir (Wien). 2005;147:587-94.
- [Google Scholar]
- Role of decompressive surgery in the management of severe head injuries: Prognostic factors and patient selection. J Neurotrauma. 2005;22:1311-8.
- [Google Scholar]
- Decompressive bifrontal craniectomy for malignant intracranial pressure following anterior communicating artery aneurysm rupture: Two case reports. Neurocrit Care. 2007;6:49-53.
- [Google Scholar]
- Posterior fossa decompression and clot evacuation for fourth ventricle hemorrhage after aneurysmal rupture: Case report. Neurosurgery. 2001;49:208-11.
- [Google Scholar]
- Decompressive hemicraniectomy for the treatment of intractable intracranial hypertension after aneurysmal subarachnoid hemorrhage. Stroke. 2007;38:987-92.
- [Google Scholar]
- Decompressive hemicraniectomy for poor-grade aneurysmal subarachnoid hemorrhage patients with associated intracerebral hemorrhage: Clinical outcome and quality of life assessment. Neurosurgery. 2005;56:12-9.
- [Google Scholar]
- Role of controlled lumbar CSF drainage for ICP control in aneurysmal SAH. Acta Neurochir Suppl. 2011;110:183-7.
- [Google Scholar]






