Translate this page into:
Clinical profile of parkinsonian disorders in the tropics: Experience at Kano, northwestern Nigeria
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
No data exists on Parkinson's disease (PD) and secondary Parkinsonism in Northwestern Nigeria. This study was designed to create a database, document the clinical profile of PD in Kano, northwestern Nigerian, and compare this to prior observations within and outside Nigeria.
Materials and Methods:
A database was documented on prospective patients presenting consecutively to the Neurology out-patients clinic of the two tertiary health facilities in Kano northwestern Nigeria over a period of 4 years. Demographic and clinical data at presentation were documented for all patients. Cases were classified as PD or secondary Parkinsonism. The severity at presentation and at last visit was classified using the H and Y scale.
Results:
Over a period of 4 years, out 1153 a total of 96 patients comprising 74 males and 22 females were enrolled. Eighty (83.3%) of them had clinically diagnosed PD while 16 (16.7%) had clinical features compatible with secondary Parkinsonism. The mean age at onset of symptoms in the PD patients (mean 58.2 ± 6.72 yrs ) was more than in secondary Parkinsonism (mean 51.4 ± 10.04 and P = 0.001). There was male preponderance in both idiopathic Parkinsonism (PD) (m:f = 3.2:1) and secondary Parkinsonism (m:f = 4.3:1). Out of the patients with secondary Parkinsonism, 10 (62.5%) and 5 (31.3%) had vascular Parkinsonism and drug-induced Parkinsonism, respectively. Duration of symptoms prior to presentation ranged between 3 months and 16 years. The mean (SD) time interval from the onset of motor symptoms to diagnosis of PD was 3.6 ± 3.4 yrs and time interval for men and women (male 3.8 ± 3.7; female 2.8 ± 2.1; P = 0.249).
Conclusions:
Clinical profile of patients with PD and secondary Parkinsonism in Kano is similar to that from other populations within Nigeria and other developing countries. However, delayed presentation, less frequent family history, lower frequency of Young-onset PD as well as treatment challenges occasioned by poverty, inadequacy of expert, and lack of newer drugs and treatment options contrasts the situation in western populations.
Keywords
Kano
Parkinson's disease
Introduction
Parkinsonism is a movement disorder characterized by tremors, rigidity, and bradykinesia and postural instability. Parkinson's disease (PD), the idiopathic form of Parkinsonism, was said to occur less frequently in populations of African origin than elsewhere in the world.[1] In western Nigeria, the prevalence rate is between 50 and 90 per 100,000 of the population aged above ten years[23] and age specific rate of 67 per 100,000 in a rural community in southwestern Nigeria.[4] The lower prevalence in comparison to both the black and Caucasian population in industrialized western countries such as the United States of America has been attributed to possible genetic, environmental or demographic factors[4] Previous studies on Parkinsonism and PD in Nigeria,[56] mostly from Southwestern Nigeria, provided data comparable to what obtains in the other developing countries of the world.
Nigeria is a vast country with varied ethnicity, numerous languages, great cultural diversity, and possibly large genetic pool. In spite of this, a majority of the old and recent published data on Parkinsonism originated from southwestern Nigeria[2–6] with very few or none from northwestern Nigeria.
We therefore relate our experience in respect of the clinical spectrum and management of this syndrome from Kano, northwestern Nigeria.
Materials and Methods
At the Aminu Kano Teaching Hospital (AKTH) and Murtala Muhammad Specialist Hospital (MMSH), Kano, Nigeria, a database of patients presenting to the Neurology out-patient clinics was established from June 2007 to June 2011. The two hospitals are the two tertiary health facilities in Kano, the most populous state in Nigeria.[7] Demographic and clinical data of all consecutively attending patients with Parkinsonism over a 4-year period were systematically documented. A proforma was completed for each patient documenting the diagnosis of PD, demographic data as well as the patient characteristics including the age at onset of the disease, age at presentation, and initial symptoms and distribution of symptoms of PD. Young onset PD was acceptable if the age at onset of the illness £ 50 years.
The diagnosis of Parkinsonism was based on the presence of at least three of the four cardinal features of tremors, rigidity, bradykinesia, and postural or gait abnormality.
Only patients whose diagnosis was based on presence of at least three of four cardinal clinical features (tremor, rigidity, bradykinesia, and postural instability) and response to levodopa therapy were said to have PD. Also required for the diagnosis of PD were: absence of identifiable cause, absence of atypical features like preceding or early cognitive impairment, early or preceding dysautonomia, unexplained corticospinal tract or cerebellar features.[6]
Diagnosis of secondary Parkinsonism was based on the constellation of clinical features suggestive of a secondary etiology[8–10] and neuroimaging (Brain MRI and CT scan) evidence of brain lesion which is temporally and anatomically related with the diagnosis of Parkinsonism.
The severity at presentation and last visit (6months to 4 years from onset of the illness) was classified using the Hoehn and Yahr (H and Y) scale.[1112]
All the patients were evaluated by a qualified neurologist during the course of their illness.
Data were analyzed using SPSS version 17 statistical software. Continuous data are expressed as mean (standard deviation) (SD), median, and ranges. Mean values were compared using the Student t test. The χ2 test was used to compare categorical variables which were presented as percentage. A P-value < 0.05 was considered as statistically significant.
Results
Over a period of 4 years, out of 1153 patients reviewed in neurology clinic of the two hospitals, a total of 96 (8.3%) eligible patients comprising 74 males and 22 females with Parkinsonism were recruited. Eighty (83.3%) of them had clinically diagnosed PD while 16 (16.7%) had clinical features compatible with secondary Parkinsonism. The demographic characteristics of the patients are as shown in Table 1. Thirty-six (45%) and seven (43.8%) of those with PD and secondary Parkinsonism respectively were in the fifth decade of life. Only ten (12.5%) of the patients with PD presented before the age of 40 yrs. Most common occupations of the patients before retirement were civil service (40%), farming (25%), and trading (22.5%). Comparing the two classes of Parkinsonism, the mean age at onset of symptoms in the PD patients (mean 58.2 ± 6.72 yrs) was higher than in secondary Parkinsonism (mean 51.4 ± 10.04) and the difference was statistically significant (P = 0.001). There was a male preponderance in both idiopathic PD (m: f = 3.2:1) and secondary Parkinsonism (m:f = 4.3:1).
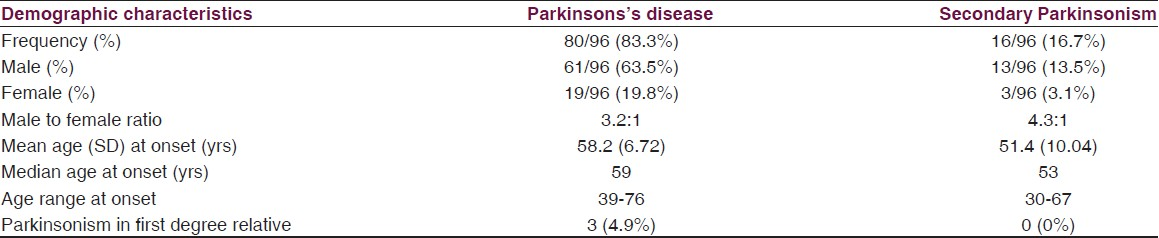
The clinical features at presentation were as reflected in Table 2. The clinical phenotype was tremor dominant in (46%) and akinetic-rigid dominant in 42%. The first motor symptom was tremor of the hands in 60 (75%) PD patients and the motor symptom started on one side of the body in 70 (87.5%) patients. A positive family history of Parkinsonism was present in only 4.9% of PD cases and 0% of secondary Parkinsonism.

Out of the patients with secondary Parkinsonism, ten (62.5%) had vascular Parkinsonism, five (31.3%) had drug-induced Parkinsonism, and one (6.3%) had head trauma-related Parkinsonism.
Duration of symptoms prior to presentation was quite variable, ranging from 3 months to 16 years. The mean (SD) time interval from the onset of motor symptoms to diagnosis of PD was 3.6 ± 3.4 yrs and there was no statistically significant difference between time interval for men and women (male 3.8 ± 3.7; female 2.8 ± 2.1; P = 0.249, 95% C.I. = –0.749 to 2.850).
Only 10 (12.5%) of the patients had received a definite diagnosis of PD prior to specialist consultation.
A majority (38.8%) of the subjects with PD were in Stage 2 (Hoehn and Yahr) at presentation. The distribution of PD cases by disease severity at presentation is as shown in Table 3.

All the 80 PD patients were placed on levodopa/carbidopa combination. They all had 250 mg/25 mg, which happened to be the only strength available in our setting, starting at 1/2 tablet (125 mg levodopa/12.5 mg carbidopa) 8 hourly with individualized upward titration and dose adjustments tailored to clinical response in each case. Fifty (62.5%) were on benzhexol with most challenging side effect being confusion. Thirty (37.5%) also had dopamine agonist comprising bromocriptine (20 patients), pramipexole (8 patients), and ropinirole (2 patients). Two patients had selegiline.
Within a 4-year study period, clinical fluctuation was documented in 21 patients (26.3%) and dyskinesia in 14 (17.5%) PD patients, 2 deaths were reported, 5 of the patients were lost to follow-up and only 2 patients are being prepared for surgery in India (deep brain stimulation/lesioning). Throughout the study, only two patients were admitted, one for frequent freezing episodes and the other for sepsis from chest infection.
Discussion
PD is said to be less common in sub-Sahara African populations than elsewhere in the world,[13] our experience in northwestern Nigeria, like in southwestern Nigeria,[6] is in the contrary as one out of every twelve patients we see in Neurology clinics in the two tertiary centers in Kano, northwestern Nigeria, has PD.
Like in many other developing countries, PD is probably an underdiagnosed disease in Nigeria. This is because all the major clinical features of the disease, such as tremors, slowing of movements, and gait as well as posture abnormalitie are considered as features of normal ageing by the general population as they may never seek medical advice and hence, the diagnosis may not be made.[14] Nevertheless, because the study was hospital based, bias in terms of actual number and spectrum of cases encountered as well as severity of PD and secondary Parkinsonism in the general population is not absolutely unlikely. It is also worth bearing in mind that the issue of general practitioner referral behavior, patterns and rates is a highly complex area in our setting Patients in this study belonged to different socio-economic class, some of them passed through the primary and secondary care centers to get to the study tertiary centers giving room for referral filter bias.. The demographic parameters of our patients are similar, with minimal variation, to that reported in the earlier study in southwestern Nigerian.[56] The mean age at onset in the present study was 58.2 and 51.4 years for PD and secondary Parkinsonism respectively. Okubadejo and colleagues reported mean age at onset of the illness of 61.5 and 57.5 respectively in southwestern Nigeria[6] . However, these figures were higher than the 55.6 years reported by Osuntokun et al.[5] also in Southwestern Nigeria. A similar finding had been reported elsewhere.[14–18]
The gender difference in PD patients (m:f = 3.2:1) seen in this study is similar to the finding (m:f = 3.2: 1) of Okubadejo and colleagues in Lagos as well of that of Osuntokun et al. (m:f = 4.2 :1)[4] southwestern Nigeria.[6] Male preponderance was also recorded in Pakistan (1.7: 1)[14] and China where PD was found to be three times more common in men.[18] An epidemiological study from India revealed that men were at increased risk of developing PD (OR = 1.98; 95%, C.I. = 1.34-2.92).[19] This finding is, however, contrary to what obtains in some western literature, where the disease affects males and females almost equally.[18]
In the current study, PD accounting for a significantly higher proportion of Parkinsonism is divergent from the finding of Osuntokun and colleagues in Ibadan, Southwestern Nigeria three decades ago,[5] however, Okubadejo and colleagues,[6] in a more recent study in Lagos, southwestern Nigeria, also reported a significantly higher proportion of PD. This inconsistency between the old and new studies in Nigeria might partly be a reflection of more recent improvements and streamlining of the diagnostic criteria for PD and other parkinsonian syndromes in both the clinical and research arena in contrast to the status at the time of the earlier report.[56] On the other hand, improvement on some of the risk factors for secondary Parkinsonism like availability of atypical antipsychotics may also have contributed to the discrepancy observed.
Late presentation recorded in this study is in keeping with the findings of other workers in and outside Nigeria.[561416] This delay in seeking medical consultation could be partly attributed to insidious onset as well as gradual progression of the disorder and partly ascribed to erroneous presumption on the part of the patients and their relations that the clinical manifestations are solely age-related events. Besides, in the present study, only 10% of the patients enrolled for the study had the right diagnosis of Parkinsonism; thus, poor recognition of the cardinal features of Parkinsonism and of the existence or benefit of available therapies in alleviating the symptoms and improving the quality of life of people with PD might have contributed to late referrals.[6] It has also been shown in previous reports that the elderly blacks were less likely to seek medical attention than other races.[20]
Generally, the clinical features profile of PD and secondary Parkinsonism seen in the present study do not appear to vary substantially from that reported in other populations with tremor, rigidity, and bradykinesia as the most common manifestations. In this study, none of the patients was a blacksmith, an occupation which was previously reported by Osuntokun et al. as a possible risk factor for PD.[5] We found a positive family history of PD in a first degree relative in three of our patients, two out of whom had young onset PD, they developed PD below 40 years of age. However, in a preliminary analysis of the genetic contributions to PD among Nigerians which explored the role of mutations in LRRK2, PRKN, and ATXN3 in apparently sporadic PD compared to age - and ethnically-matched controls, common pathogenic mutations in these genes, previously observed in several populations, are not frequent causes of PD in Nigerians.[21]
One out of every eight patients enrolled in our study repeatedly complained of forgetfulness. Although detailed cognitive assessment was not carried out on this cohort, cognitive impairment among patients with PD had been previously reported in a study by Akinyemi et al. in Nigeria. In that study, they showed that cognitive dysfunction occurred more frequently in Nigerians with PD compared to controls and that older age at disease onset was an important determinant of cognitive dysfunction in PD.[22]
Presently, anti-parkinsonian drugs that are readily available in the region are limited to levodopa/carbidopa, bromocriptine, and benzhexol. Few of the patients were on ropinirole, pramipexole and selegiline individually sourced theses from India, Egypt, and Saudi Arabia. This is a true reflection of the report that medications for PD in Africa were available to only 12.5% of those who needed them, compared to 79.1% in Europe.[20]
One of the major challenges in the management of these patients is poor drug compliance occasioned by financial constraints the treatment is a private responsibility. The drugs that are available are manufactured outside the country and are also expensive but a majority of the affected patients are farmers and petty traders who live in villages and suburban communities with a large number of them under the poverty line.
Most patients are apprehensive about the side effects particularly dyskinesia and confusion caused by benzhexol; thus, it is not uncommon to come across patients who unilaterally reduce the dose of the drugs or even stop taking them altogether. Therefore, more effort is being intensified in the area of education in respect of the disease progression as well as the benefit and adverse effects of the medications.
Any new drug that is tested internationally takes decades to become available to Nigerian patients through importation. Surgical treatment for PD (e.g. deep-brain stimulation and lesioning) is not available in any center in the country therefore those that are candidates for surgery and can afford the procedure are referred abroad.
The modest clinical improvement recorded on follow-up of our patients for 6 months to 4 years may be a mere symptomatic effect, rather than an effect on disease progression. Throughout the study, only two patients were admitted, one for frequent freezing episodes and the other for sepsis. In a previous study in Kano, PD was shown to be an uncommon cause of neurological admission.[23]
Conclusion
Our study from Kano, northwestern Nigeria, provides data on the clinical profile of patients with PD and secondary Parkinsonism which is largely similar to that from other populations within Nigeria and other developing countries. However, compared with data from southwestern Nigeria, the mean age at onset is slightly lower and family history of PD slightly higher. In comparison with the old data in Nigeria, a higher proportion of PD (of all Parkinsonism) was recorded. Moreover, delayed presentation, less frequent family history, lower frequency of Young-onset PD as well as treatment challenges occasioned by poverty, inadequacy of expert and lack of newer drugs and treatment options contrasts the situation in western populations.
Source of Support: Nil
Conflict of Interest: None declared
References
- Research protocol for measuring the prevalence of neurologic disorders in developing countries: Results of a pilot study in Nigeria. Neuroepidemiology. 1982;1:143-53.
- [Google Scholar]
- Neurological disorders in Nigerian Africans: A community-based study. Acta Neurol Scand. 1987;75:13-21.
- [Google Scholar]
- Comparison of the prevalence of Parkinson's disease in black populations in the rural United States and in rural Nigeria: Door-to-door community studies. Neurology. 1988;38:645-6.
- [Google Scholar]
- Parkinsonism in the Nigerian African: A prospective study of 217 patients. East Afr Med J. 1979;56:597-607.
- [Google Scholar]
- Clinical profile of Parkinsonism and Parkinson's disease in Lagos, Southwestern Nigeria. BMC Neurol. 2010;10:1.
- [Google Scholar]
- National Population Commission. List of Nigerian State by Population. Federal republic of Nigeria Census 2006 http://www.population.gov.ng
- [Google Scholar]
- Multiple system atrophy - the nature of the beast. J Neurol Neurosurg Psychiatry. 1989;52:78-89.
- [Google Scholar]
- Diagnosis and differential diagnosis of Parkinson's disease and Parkinsonism. Parkinsonism Relat Disord. 2001;7:63-70.
- [Google Scholar]
- How valid is the clinical diagnosis of Parkinson's disease in the community? J Neurol Neurosurg Psychiatry. 2002;73:529-34.
- [Google Scholar]
- Movement Disorder Society Task Force on Rating Scales for Parkinson's Disease. Movement Disorder Task Force report on the Hoehn and Yahr staging scale: Status and recommendations. Mov Disord. 2004;19:1020-8.
- [Google Scholar]
- Community-based study of neurological disorders in rural central Ethiopia. Neuroepidemiology. 1990;9:263-77.
- [Google Scholar]
- Clinical spectrum of Parkinson's disease from Pakistan. Singapore Med J. 2006;47:1075.
- [Google Scholar]
- Prevalence of Parkinson's disease in Parsi community in Bombay, India. Arch Neurol. 1988;45:1321-3.
- [Google Scholar]
- Parkinson's disease in Ethiopia: A prospective study of 70 patients. East Afr Med J. 1985;62:571-9.
- [Google Scholar]
- Risk factors of Parkinson's disease in Indian patients. J Neurol Sci. 2001;190:49-55.
- [Google Scholar]
- The challenge of Parkinson's disease management in Africa. Age Ageing. 2007;36:122-7.
- [Google Scholar]
- Analysis of Nigerians with apparently sporadic Parkinson disease for mutations in LRRK2, PRKN and ATXN3. PLoS One. 2008;3:e3421.
- [Google Scholar]
- Cognitive dysfunction in Nigerians with Parkinson's disease. Mov Disord. 2008;23:1378-83.
- [Google Scholar]
- Pattern of neurological admissions in the tropics: Experience at Kano, northwestern Nigeria. Ann Indian Acad Neurol. 2010;13:167-70.
- [Google Scholar]






