Translate this page into:
Correlative study between neuron-specific enolase and blood sugar level in ischemic stroke patients
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
A study to investigate the level of the neurobiochemical marker, Neuron-Specific Enolase (NSE), at the time of admission and its correlation with the blood sugar level in ischemic stroke patients.
Patients and Methods:
We investigated 90 patients with complete stroke who were admitted to the Stroke Unit of the Department of Neurology at Sri Aurobindo Institute of Medical Sciences. NSE was measured with commercially available quantitative ‘sandwich’ enzyme-linked immunosorbent assay kits obtained from R and D Systems. Hyperglycemia was defined as blood glucose concentration ≥ 7 mmol / L, and measured using the glucose oxidase method immediately.
Results:
Significantly increased NSE and lipid profile levels were found in ischemic stroke patients as compared to the control. Hyperglycemic ischemic stroke patients had increased levels of NSE, lipid profile, and National Institute of Health stroke scale scores (NIHSS score) compared to normoglycemic ischemic stroke patients. In addition the serum NSE level of hyperglycemic stroke patients was also positively correlated with the blood sugar level (r = 0.734 P < 0.001).
Conclusions:
Hyperglycemia predicts an increased risk of poor outcome after ischemic stroke and it is reflected by a significantly increased level of Neuron-Specific Enolase.
Keywords
Hyperglycemia
Ischemic Stroke
lipid profile
neuron-specific enolase
Introduction
Elevated blood glucose is common in the early phase of stroke. The prevalence of hyperglycemia, defined as blood glucose level > 6.0 mmol / L (108 mg / dL), has been observed in two thirds of all ischemic stroke subtypes on admission, and in at least 50% in each subtype including lacunar strokes.[1] The recent experimental studies add that hyperglycemia aggravates edema formation in the zone surrounding cerebral hemorrhages.[2] Other studies have also shown that hyperglycemia in ischemic stroke is associated with poor outcome,[34] however, it remains uncertain whether hyperglycemia directly contributes to the worsening of ischemic stroke.
Neuron-specific enolase (NSE) is present in high concentrations in neurons, where it catalyses the conversion of 2-phosphoglycerate into phosphoenolpyruvate. NSE is released into the cerebrospinal fluid and blood, in response to different forms of brain injury, including ischemic stroke, and can serve as a peripheral indicator of the ongoing neuronal damage.[5–8]
Many studies have provided strong evidence for lipids as a risk factor for coronary artery disease (CAD). These studies demonstrate a direct relationship between total cholesterol, low-density lipoprotein (LDL), and CAD, and an inverse relationship between high-density lipoprotein (HDL) and CAD.[9–11] These relationships are not yet clearly established for ischemic stroke and some studies even question whether cholesterol is a risk factor for stroke or not.
We therefore investigated a difference in serum NSE concentration and lipid profile between stroke patients and healthy control, followed by comparing serum NSE levels, lipid profiles, and the National Institute of Health Stroke Scale (NIHSS) in patients with acute ischemic stroke, with and without increased blood glucose concentrations.
Patients and Methods
Patients
We consecutively included 90 patients, 60 men and 30 women with their first-ever ischemic stroke. They were admitted within 72 hours of the onset of stroke symptoms to the Stroke unit of the Department of Neurology, Sri Aurobindo Hospital, Indore, MP. All the patients were treated according to the guidelines of the American Heart Association and none of them underwent surgical procedures. Our exclusion criteria were (1) CSF Infection (2) Stroke of more than 72 hours (3) Peripartum stroke, and (4) Head Trauma. The study protocol was approved by the appropriate institutional Ethical Committee and informed consent was obtained from all the study participants. We also enrolled a group of 101 control individuals with no history of stroke, who had admitted to our hospital for routine checkup. Some controls were recruited from the hospital staff.
Methods
Blood samples were collected at the time of admission. The patients blood was then centrifuged, serum samples separated, aliquoted, and kept frozen at - 20°C, prior to analysis. NSE was measured with commercially available quantitative ‘sandwich’ enzyme-linked immunosorbent assay kits obtained from the R and D Systems. Sensitivity of the assay was 1 μg / L for NSE. Hyperglycemia was defined as blood glucose concentration ≥ 7 mmol / L, and measured by the Glucose oxidase method, immediately. The degrees of neurological deficit during the acute phase were evaluated by National Institute of Health Stroke Scale at the time of admission.
Statistical analysis
The results were presented as mean ± SD values. Each distribution was tested for normality using the Kolmogorov–Smirnov test, prior to any further analysis. Significance of age difference between the groups was tested using the parametric Student's t test. Statistical significance of the difference between the categorical variables was tested with the Chi-square test. The correlations were evaluated by using the regression analysis with the Pearson's coefficient. Only P-values ≤ 0.05 were considered significant. Datafrom different groups were analyzed by the parametric Student's t test.
Results
The demographic and clinical profiles of all the subjects (Ischemic stroke) and control did not differ significantly with regard to age (59.71 ± 12.6 vs.61.31 ± 12.37, P = 0.375) and sex as shown in Table 1.
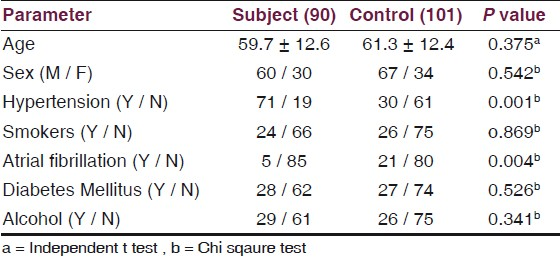
Table 2 shows the significant increased level of Neuron-Specific Enolase (NSE) in ischemic stroke patients, as compared with control (18.0 ± 4.5 vs. 7.5 ± 1.5 P = 0.001). Ischemic stroke patients also showed statistically significant increased levels of LDL (170.7 ± 28.7 vs. 88.4 ± 13.6, P = 0.005), TG (190.4 ± 32.6 vs. 116.7 ± 34.4, P = 0.003), and decreased level of HDL (31.9 ± 5.2 vs. 46.9 ± 12.1 P = 0.05), respectively, as compared to the control, shown by Figure 1.


- Comparison of lipid profile in control, normoglycemic ischemic stroke and hyperglycemic ischemic stroke
In the acute phase of brain infarction, the concentrations of NSE in the serum is significantly increased with an increase in the blood glucose levels, in the controls, Normoglycemic ischemic stroke patients, and Hyperglycemic ischemic stroke patients, respectively [Figure 2].

- Box plots of serum neuron-specific enolase concentrations in control, Normoglycemic Ischemic stroke patients and Ischemic stroke patients with hyperglycemia. Hyperglycemia was defined as blood glucose concentration of > 7m mol / l. Results were expressed as median values with lower and upper quartiles. Whiskers displayed non-outliner maximal and minimal values
Table 3 demonstrates a comparison between Normoglycemic Ischemic stroke patients and Hyperglycemic ischemic stroke patients. Hyperglycemic ischemic stroke patients had increased levels of NSE (19.7 ± 4.7 vs. 15.2 ± 2.4, P= 0.05), LDL (181.5 ± 24.0 vs. 153.7 ± 26.4, P = 0.05), TG (201.6 ± 29.4 vs. 172.9 ± 29.8, P = 0.04), Blood sugar (148.9 ± 15.4 vs. 104.6 ± 9.9 P = 0.001), and NIHSS score (15.6 ± 6.8 vs. 10.2±6.6, P = 0.003), with a significant decreased level of HDL (30.4 ± 5.3 vs. 34.4 ± 4.0, P = 0.005), as compared to Normoglycemic ischemic stroke patients.

Serum NSE level in Hyperglycemic stroke patients was also found to be positively correlated with the blood sugar level (r = 0.73 P < 0.001) shown in Figure 3.

- Correlation between Neuron-specific enolase concentration ng / ml and Blood sugar level mg%. r = correlation coefficient, P < 0.001 statistically significant
Discussion
Neuron-specific enolase is a soluble protein enolase enzyme (2-phopho-D-glyceride hydrolase) of the glycolytic pathway, with a total molecular weight of approximately 80000 daltons.[12] It counts 1.5% of cell-soluble brain proteins and is found predominantly in neurons and neuroendocrine cells.[13] After various types of insults in the central nervous system, such as, cerebral infarction, hypoxia trauma, and seizure, the blood brain barrier gets disturbed, and substantial astroglial disintegration makes the NSE leak into the cerebrospinal fluid and serum.[14] It is mentioned as a possible reliable marker of neuronal tissue damage.[15] We evaluated the serum NSE level rather than the CSF level, because the daily serum sampling was practical and posed no risk for older patients.
In the previous reports, the levels of NSE in the serum peaked within the first 96 hours of cerebral infarction, and in some cases as late as day six after infarction.[16–21] The half-life of NSE in the serum has been reported to be about 48 hours,[22] hence, the serum levels of NSE will be expected to rise as long as damage due to the infarction continues and NSE is washing out of the brain tissue. The time to the peak serum level of NSE in our study was 72 hours after infarction, which compares well with the 48-hour half-life reported in the literature. Our data show highly significant increased admission NSE levels in stroke patients as compared to the control group. The increased NSE serum levels correspond to the ischemia-induced cytoplasm loss of NSE in the neurons and are detectable before irreversible neuronal damage takes place.[22]
A conspicuous finding of the present study that the concentration of serum NSE levels in hyperglycemic stroke patients was significantly more than those in the normoglycemic stroke patient group, adds further support to the concept that hyperglycemia enhances neuronal necrosis, and hyperglycemia-induced lactic acidosis in the ischemic brain not only damages glial and endothelial cells, but may also exacerbate the biochemical events in the ischemic penumbra that lead to neuronal cell death and release of biochemical markers, shown by the positive correlation between NSE and the blood sugar level [Figure 2] during the acute stage of ischemic stroke. One study has shown that hyperglycemia in patients with pure motor stroke, due to lacunar infarctions, is not associated with increased NSE levels.[23] The problem of hyperglycemia in acute stroke is important, as it occurs in about 20% of non-diabetic patients.[23] The mechanism is not entirely clear, but one hypothesis is that it results from a neuroendocrine stress response.[2425]
Ischemic stroke is a heterogeneous pathophysiological entity with vastly different pathways, leading to indistinguishable clinical presentations. Well-recognized mechanisms of ischemic stroke include cardiac or artery-to-artery embolism, atherothrombosis of an extracranial carotid or intracranial artery, and nonatherosclerotic disease of small diameter penetrating arteries.[26] The lipid profile might have a more important role in those ischemic strokes that are the consequence of atherosclerosis of larger arteries.[27] In our study Low Density Lipoproteins (LDL) and Triglycerides (TG) increased with a significantly decreased level of High Density Lipoproteins (HDL), which is supported by several other studies.[28–31] Previous studies have shown that elevated LDL is a risk factor for vascular disease and high levels of HDL are protective.[3233] One study has demonstrated that an association between post stroke lipids and prognosis may vary by sex. In women, lipids were not associated with the outcome; in men, a higher level of TG and LDL were associated with worse prognosis.[34] The mechanism of lipid changes remains unclear, but it is thought to relate in part to the stress and associated catecholamine overproduction of an acute stroke.[35] Baseline lipid panel components have not been associated with an increased stroke risk in one cohort study, hence, treatment with cholesterol-lowering medications and lipid measurements at several points may be better markers of stroke risk.[36]
Source of Support: Nil
Conflict of Interest: None declared.
References
- Prevalence of admission hyperglycemia across clinical subtypes of acute stroke. Lancet. 1999;353:376-7.
- [Google Scholar]
- Spreading depression-induced gene expression is regulated by plasma glucose. Stroke. 1999;30:114-9.
- [Google Scholar]
- Glucose modulation of ischemic brain injury: Review and clinical recommendations. Mayo Clin Proc. 1996;71:801-12.
- [Google Scholar]
- Is hyperglycaemia an independentpredictor of poor outcome after acute stroke.Results of a long-term follow up study. Br Med J. 1997;314:1303-6.
- [Google Scholar]
- Serum neurone-specific enolase as an indicator of stroke volume. Eur J Clin Invest. 1996;26:298-303.
- [Google Scholar]
- S-100 protein and neuron-specific enolase concentrations in blood as indicators of infarction volume and prognosis in acute ischemic Stroke. Stroke. 1997;28:1956-60.
- [Google Scholar]
- S-100 protein and neuron-specific enolase in cerebrospinal fluid and serum: Markers of cell damage in human central nervous system. Stroke. 1987;18:911-8.
- [Google Scholar]
- Neuron-specific enolase concentrations in blood as a prognostic parameter in cerebrovascular diseases. Stroke. 1994;25:558-65.
- [Google Scholar]
- Influence of low HDL on progression of coronary artery disease and response to Fluvastatin therapy. Circulation. 1999;99:736-43.
- [Google Scholar]
- Plasma high-density lipoprotein concentration and development of ischemic heart disease. Lancet. 1975;1:16-20.
- [Google Scholar]
- Measurement of neuron-specific enolase (NSE) and non-neuronal (NNE) isoenzymes of enolase in rat, monkey and human nervous tissue. J Neurochem. 1979;33:319-29.
- [Google Scholar]
- Highly sensitive immunoassays for three forms of rat brain enolase. J Neurochem. 1981;36:793-7.
- [Google Scholar]
- S-100 protein and neuron-specific enolase in CSF after experimental traumatic or focal ischemic brain damage. J Neurosurg. 1989;71:727-31.
- [Google Scholar]
- The increase of neuron-specific enolase in cerebrospinal fluid and plasma as a marker of neuronal damage in patients with acute brain infarction. J Clin Neurosci. 2005;12:542-7.
- [Google Scholar]
- Serum neurone-specific enolase as an indicator of stroke volume. Eur J Clin Invest. 1996;26:298-303.
- [Google Scholar]
- Neuron-specific enolase concentrations in blood as a prognostic parameter in cerebrovascular diseases. Stroke. 1994;25:558-65.
- [Google Scholar]
- Serum neuron-specific enolase, carnosinase, and their ratio in acute stroke.An enzymatic test for predicting outcome? Stroke. 1996;27:2064-8.
- [Google Scholar]
- Nervous system-specific enolase in serum as a marker for neuroblastoma. Pediatrics. 1983;72:696-700.
- [Google Scholar]
- S-100 protein and neuron-specific enolase concentrations in blood as indicators of infarction volume and prognosis in acute ischemic stroke. Stroke. 1997;28:1956-60.
- [Google Scholar]
- Neurone-specific enolase and N-acetyl-aspartate as potential peripheral markers of ischaemic stroke. Eur J Clin Invest. 1999;29:6-11.
- [Google Scholar]
- Early neurobehavioral outcome after stroke is related to release of neurobiochemical markers of brain damage. Stroke. 1999;30:1190-5.
- [Google Scholar]
- Increased serum neuron specific enolase concentrations in patients with hyperglycemic cortical ischemic stroke. Neurosci Lett. 1998;253:71-3.
- [Google Scholar]
- Is hyperglycaemia an independent predictor of poor outcome after acute stroke? Results of a long-term follow up study. Br Med J. 1997;314:1303-6.
- [Google Scholar]
- Glucose modulation of ischemic brain injury: Review and clinical recommendations. Mayo Clin Proc. 1996;71:801-81.
- [Google Scholar]
- Plasma Lipid Profi le and Incident Ischemic Stroke: The Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 2003;34:623-31.
- [Google Scholar]
- Interrater reliability of an etiologic classification of ischemic stroke. Stroke. 1995;26:46-51.
- [Google Scholar]
- Lipids and stroke: Neglect of a useful preventive measure? Cardiovasc Res. 1998;40:265-71.
- [Google Scholar]
- Risk factors for ischaemic heart disease and stroke mortality in young and old hypertensive patients. J Hum Hypertens. 1995;9:695-7.
- [Google Scholar]
- Influence of low HDL on progression of coronary artery disease and response to fluvastatin therapy. Circulation. 1999;99:736-43.
- [Google Scholar]
- High-density lipoprotein cholesterol and cardiovascular disease: Four prospective American studies. Circulation. 1989;79:8-15.
- [Google Scholar]
- Sex differences in the prognostic value of the lipid profile after the first ischemic stroke. J Neurol. 2009;256:989-95.
- [Google Scholar]






