Translate this page into:
Retromastoid-sub occipital: A novel approach to cerebello pontine angle in acoustic neuroma surgery-our experience in 21 cases
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Acoustic neuroma surgery poses significant challenges regarding definite management and preservation of hearing and the facial nerve are of great concern.
Aim:
To analyze the efficacy of the retromastoid approach in acoustic neuroma surgery.
Materials and Methods:
Tumors operated between January 2002 and December 2008, by the authors, using the retromastoid approach, were analyzed. Twenty-one patients who presented with acoustic tumor were considered for this study.
Discussion:
Precise knowledge of the neuroanatomy in the cerebellopontine angle is the key to success and microsurgical technique is the sole factor for good outcome.
Conclusion:
Retromastoid, in fact is the approach to the skull base with minimal or no damage to neurovascular structures, in contrast to the translabyrinthine or presigmoid approach.
Keywords
Acoustic neuroma
microsurgical technique
retromastoid approach
Introduction
Acoustic neuroma accounts for approximately 80% of tumors found within the cerebellopontine angle. Clinically diagnosed acoustic neuromas occur in 0.7-1.0 persons per 100,000 population. Acoustic neuromas are intracranial extra-axial tumors that arise from the Schwann cell sheath investing either the vestibular or cochlear nerve.
From the era of Cushing, which involved enucleation of the total tumor, to the modern neurosurgical technique, following adaptation of the operating microscope at the beginning of the 1960s and CUSA (Cavitron ultrasonic surgical aspirator), the cerebellopontine angle is no longer a difficult site to approach.
Materials and Methods
In this retrospective study, tumors operated between January 2002 and December 2008, by the authors, using the retromastoid approach, were analyzed. Twenty-one patients who presented with acoustic tumor were considered for this study. The outcome was assessed and compared with the technique, complication, facial nerve preservation, and extent of tumor resection.
After initial neurological assessment, patients were subjected to audiometry in all cases and BAEP (brainstem evoked response) in selected cases. Audiometric work-up was done with pure-tone air conduction, pure-tone bone conduction, and speech discrimination. In all cases contrast computed tomography (CT) and magnetic resonance imaging (MRI) of brain was performed preoperatively, and in follow-up only contrast MRI of brain was performed to document completeness of tumor resection. Tumor sizes were measured considering intra- and extrameatal tumor extension; large tumors were larger than 30 × 20 mm, and small tumors measured up to 30 × 20 mm. Tumor extension was described as follows: Class T1, purely intrameatal; Class T2, intra- and extrameatal; Class T3a, filling the cerebellopontine cistern; Class T3b, reaching the brainstem; Class T4a, compressing the brainstem; Class T4b, severely dislocating the brainstem and compressing the fourth ventricle.[1]
Retromastoid approach
The patient is placed in the supine position on the operating table; the head is turned towards the contra lateral shoulder and fixed with Mayfield head clamp. Facial nerve electrodes are placed around the orbicularis oris and oulis muscles and are connected with the monitor. The operation is performed through a vertically oriented S-shaped incision. A suboccipital craniectomy is then realized to get sufficient room for a good, direct view to the cerebellopontine angle. The dura is opened, cisterna magna punctured, retractor placed and the arachnoid incised. The cerebellum frequently falls away from the posterior surface of the temporal bone. Once adequate exposure has been obtained, the tumor is clearly visualized along with the brainstem and lower cranial nerves. However, cranial nerves VII and VIII are rarely observed because they are almost always pushed forward and lie across the anterior surface of the tumor, which cannot be visualized. Debulking of the tumor is the next step and must be carefully performed under an operating microscope, using micro dissecting instruments, so as to maintain the anterior portions of the capsule if injury to cranial nerve VII and/or VIII are to be avoided. Once the tumor has been substantially debulked, the posterior wall of the internal auditory canal can be removed using a high-speed drill. Great care must be taken to avoid injuring the labyrinth while removing the posterior wall of the internal auditory canal. Blind extraction of tumor from the internal auditory canal without removing the posterior wall can put significant risks of injury to the facial and/or auditory nerve. Once the internal auditory canal is exposed, the dura is opened and the tumor is removed from it. The vestibular nerves are generally sacrificed, and unless hearing is to be preserved, the cochlear nerve is sacrificed as well.
Eventually, the surgeon is left with the anterior portions of the capsule adhered to the brainstem and cranial nerve VII. As the tumor capsule is carefully removed from the brainstem, the root entry zone of cranial nerve VII can be identified. The capsule is then carefully removed from the facial nerve with as little trauma as possible. The facial nerve monitoring is of great help in this portion of the dissection to preserve the facial nerve.
All the lower cranial nerves and draining veins are preserved. Good hemostasis is secured, dura closed primarily and wound closed in layers. The patient is managed postoperatively on ventilation.
Results
Out of 21 patients 13 were males and eight were females. The age ranged between 14 to 60 years with an average of 37 years. The tumor was right-sided in eight and left sided in 13 patients.
In the neurologic work-up the most common findings were elevated pure-tone air conduction thresholds, elevated pure-tone bone conduction or equal to pure-tone air conduction. Speech discrimination was found moderate to severe loss and was out of proportion to pure-tone loss . Brainstem evoked response was delayed. Most common BAEP findings are prolonged I-III and I-V inter-peak latencies. It has <5% false negatives and 85% specificity in distinguishing acoustic neuroma. The usual neuroma would present as unilateral sensorineural hearing loss with discrimination impairment out of proportion to pure-tone thresholds and positive (delayed) brainstem auditory evoked response.
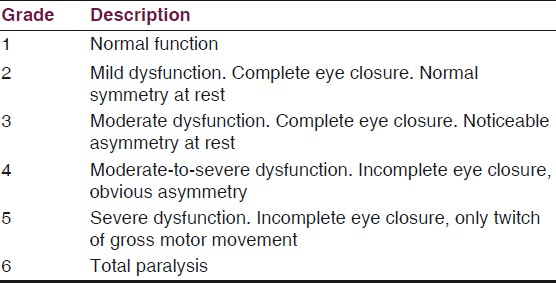
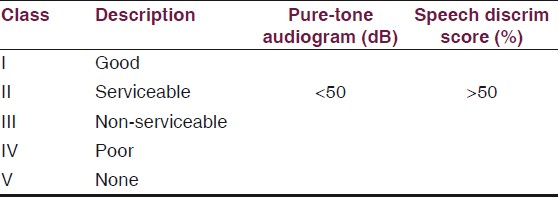
The VIIth nerve involvement was assessed with House-Brackmann Classification [Table 1] and hearing assessment was done according to Gardener-Robertson Hearing Classification [Table 2]. Facial nerve involvement was Grade-1 in one patient, Grade-4 in ten patients, Grade-5 in six and Grade-6 in four patients. Serviceable hearing was noted in two patients only.
In all the patients, the tumors were larger than 30 × 20 mm. The size of the tumor was Class T3a and was filling the cerebellopontine cistern in two patients . In Class T3b, the tumor was reaching the brainstem in nine patients. Similarly, in Class T4a, the tumor was compressing the brainstem in six cases and in Class T4b, severely dislocating the brainstem and compressing the fourth ventricle in four patients. The T4b, four patients underwent VP (ventriculo-peritoneal) shunt prior to definite surgery.
Complete tumor resection was achieved in 18 cases and tumors attached to the IAM (Internal acoustic meatus) were left behind in three cases, which were subjected to Gamma knife subsequently.
The facial nerve was preserved in all cases and postoperative deficit was unchanged in 11 patients, worsened in nine (Grade 4 to 6) and no deficit (Grade 1) was observed in one case. Hearing recovery was achieved in follow-up at the end of one year, in two patients only. In one case facial- hypoglossal anastomosis was carried out after one year. The postoperative mortality was two in number. Statistical data analysis was not done due to the small number of cases.
Discussion
The operative mortality rate has dropped dramatically from 40% at the beginning of the century to less than 1-2% in the last decade. Postoperative facial paralysis, once the rule, is now uncommon permanent sequelae of acoustic tumor surgery. Attempts at hearing conservation, unimaginable at the beginning of the century, are increasingly successful.
Cohen was one of the first and few who reported on the indications for using the retromastoid approach for preservation of hearing with only 8% facial nerve paralysis and no mortality.[23] Microsurgical treatment is one of the promising directions in modern neurosurgery.[4]
Glasscock et al., reported on the retromastoid and middle fossa approaches in 161 selected patients. They found a lower incidence of temporary facial nerve paresis using the retromastoid approach.[5] Mazzoni et al., reported on a selected series of 90 of 300 patients operated on using the retromastoid approach for a trial of preservation of hearing. The facial nerve was preserved in 99%, with completely normal function in 78%. The cochlear nerve was anatomically preserved in 96% and functionally in 44%.[6] However, facial nerve outcomes do continue to vary according to tumor size. When tumors are smaller than 1.5 cm, good facial nerve function can be expected (House-Brackmann Grade I-II) in more than 90% of patients. Only 3.2-6.7% of patients with this size of tumor have poor outcomes (House-Brackmann III-IV). The facial nerve was preserved in all cases. Postoperative deficit was unchanged in 11 patients (52%), and worsened in nine (42%) (House-Brackmann V-VI) patients. In one patient there was no facial nerve involvement. This higher grade facial nerve paresis in this study is possible due to late report ing of patients to our institution.
By the retromastoid approach, 979 tumors were completely removed by Samii et al., anatomic preservation of the facial nerve was achieved in 93% of the patients and of the cochlear nerve in 68%. The current treatment options of complete tumor resection with ongoing reduction of morbidity are well fulfilled by the retromastoid approach.[1]
In a series of 62 acoustic neuromas, 22 patients had usable preoperative hearing. Thirty tumors were less than 2.5 cm in diameter and 32 greater in size. Anatomical preservation of facial nerve was possible in 98%.[7] In summary, preservation rates by the retromastoid approach are reported to be higher than 90%, independent of tumor size, but, in general, these reports include tumors larger than 30 mm in diameter.[8]
Facial nerve paralysis may be delayed and may develop within a few hours to a week or more after acoustic neuroma removal. Incidence of delayed facial palsy varies from 10-30%. The vast majority of individuals who have delayed onset of facial paralysis make complete recoveries. If facial nerve involvement is more than 3 House-Brackmann grades , some chance of poor long-term outcome exists.
Following the introduction of improved neurophysiological monitoring techniques, the results are better. A variable amplitude discriminator rejects baseline EMG (Electro myogram) (> 50 microV) and a gating circuit prevents stimulus artifact, during stimulation from causing interference.
Auditory brainstem implants bring a new chance of hearing after tumor removal in patients with NF(Neurofibromatosis)2 .[9] In our study, preservation of hearing was possible in two cases only, not in other cases maybe due to the large size of the tumors. Hearing preservation of < 50 dB in patients with preoperative hearing threshold < 50 dB and tumors < 2.5 cm in size was 3/11 (27%). Acoustic nerve preservation should be attempted in all cases with measurable hearing, regardless of tumor size.[7] Out of 35 cases of unilateral acoustic neurinomas, operations were performed via the retromastoid approach in all cases. The facial nerve was anatomically preserved in all cases. On the other hand, the cochlear nerve was anatomically preserved in 14 out of 35 cases (40%).[10]
Opinions vary considerably about what constitutes useful hearing. The rule suggests that individuals with a pure-tone average greater than 50 dB and speech discrimination less than 50% do not have useful or salvageable hearing. Other surgeons have stricter criteria and consider only individuals with better than a 30-dB pure-tone average and more than 70% discrimination, for hearing conservation operations. Normal preoperative BAEP findings favor hearing conservation. Marked abnormalities of BAEP wave morphology or increased wave I-III and I-V latencies make hearing conservation less feasible. Opportunities for conservation of hearing decrease as tumors become larger. Hearing is much more difficult to conserve when tumors are 1.5-2.0 cm in diameter than if they are small intracanalicular tumors. Consequently, some surgeons limit hearing conservation surgery to smaller tumors, preferring to use a translabyrinthine approach to maximize the chance of facial nerve conservation for larger lesions.
Regarding the safety of the brainstem, the technique of dissection under continuous irrigation is especially helpful because cauterization may be reduced to a minimum. Identification and control of vascular supply to the brainstem are most reliably provided by the retromastoid approach, whereas access is limited in the middle fossa (MF) approach and the trans-labyrinthine (TL) approach.[11]
There is a general agreement that completeness of resection and preservation of the facial nerve are the major goals and they are being met at increasing rates. There is also agreement that any of the available approaches, such as the retromastoid suboccipital, the middle fossa and the translabyrinthine approaches, and their modifications, have their indications.[12]
The advantages of the retromastoid approach are that it can be applied to all acoustic tumors. The retromastoid approach provides the best wide-field visualization of the posterior fossa. The inferior portions of the cerebellopontine angle and the posterior surface of the temporal bone anterior to the porus acusticus are much more clearly observed than in the translabyrinthine approach.
The disadvantages of this approach are that it may require cerebellar retraction, and manipulation of the cerebellum provides opportunities for postoperative edema, hematoma, infarction, and bleeding. Its only limitation in this respect is its inapplicability for small tumors that occupy the far-lateral portions of the internal auditory canal.
Recurrence is uncommon after acoustic tumor removal. Overall, the recurrence rate is 5-10% or lower. The desired mortality rate is below 1%; the current mortality rate is approximately 1–2% and, rarely, 1–3%.[13] In this series two patients (9%) died due to associated medical problems, reflecting higher mortality, but may be due to the small number of cases and it is not related to the surgical technique or complications.
Conclusions
Retromastoid suboccipital, in fact is the approach to the skull base with minimal or no damage to neurovascular structures, in contrast to the translabyrinthine or presigmoid approach. This approach is the only one that enables preservation of hearing regardless of tumor size. Complete removal of the tumor is possible using a minimally invasive surgical technique and intraoperative nerve monitoring with a good impact on quality of life.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Management of 1000 vestibular schwannomas (acoustic neuromas): Surgical management and results with an emphasis on complications and how to avoid them. Neurosurgery. 1997;40:11-23.
- [Google Scholar]
- Acoustic neuroma surgery with emphasis on preservation of hearing. Laryngoscope. 1979;89:886-96.
- [Google Scholar]
- Surgical management of cerebellopontine angle tumors. J Otolaryngol. 1980;9:105-12.
- [Google Scholar]
- Microsurgical treatment of posterior cranial fossa tumors via keyhole approaches. Zhonghua Yi Xue Za Zhi. 2005;85:219-2.
- [Google Scholar]
- Preservation of hearing in surgery for acoustic neuromas. J Neurosurg. 1993;78:864-70.
- [Google Scholar]
- The suboccipital approach in functional surgery of acoustic neuroma. Acta Otorhinolaryngol Ital. 1993;13:3-11.
- [Google Scholar]
- Facial and acoustic nerve preservation during excision of extracanalicular acoustic neuromas using the suboccipital approach. Br J Neurosurg. 1994;8:655-65.
- [Google Scholar]
- Surgical treatment of acoustic neuromas during the last five years: Part II--Results for facial and cochlear nerve function. Surg Neurol. 1988;29:205-9.
- [Google Scholar]
- Our surgical experience with large vestibular schwannomas. Otolaryngol Pol. 2004;58:69-72.
- [Google Scholar]
- Hearing preservation and tinnitus following removal of acoustic neurinomas. No Shinkei Geka. 1996;24:329-34.
- [Google Scholar]
- Treatment of acoustic tumours in elderly patients: Is surgery warranted? J Laryngol Otol. 1993;107:295-7.
- [Google Scholar]
- Current results of the retrosigmoid approach to acoustic neurinoma. J Neurosurg. 1992;76:901-9.
- [Google Scholar]






