Translate this page into:
Post chicken pox neurological sequelae: Three distinct presentations
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Varicella zoster infection is known to cause neurological involvement. The infection is usually self-limiting and resolves without sequelae. We present a series of three cases with neurological presentations following chicken pox infection. The first case is a case of meningitis, cerebellitis and polyradiculopathy, the second is a florid case of acute infective demyelinating polyradiculoneuropathy (Guillian-Barré syndrome) in a middle-aged female and the third case is a young man in whom we diagnosed acute transverse myelitis. All these cases presented with distinct neurological diagnoses and the etiology was established on the basis of history and serological tests confirmatory for chicken pox. The cases responded differently to treatment and the patients were left with minimum disability.
Keywords
Chicken pox
meningitis
polyradiculoneuropathy
transverse myelitis
Introduction
Chicken pox is a viral infection affecting mainly children and presents with exanthematous rash with fever. The virus, after the primary infection, can remain latent in the spinal and cranial ganglia and may be reactivated at a later stage in a state of immunocompromise to present as herpes zoster.
Neurological complications following primary chicken pox infection are extremely rare (0.01–0.03%), although some neurological complications are known.[1] We report here a series of three cases demonstrating some of these neurological complications of varicella zoster virus infection.
Case Reports
Case 1
Meningitis, cerebellitis and radiculitis
A 30-year-old male presented with sudden onset weakness with inability to walk, 10 days following chicken pox infection. The weakness started simultaneously in upper and lower limbs with retention of urine and constipation. There was uncontrollable shaking of hands whenever he attempted any movement. He had dysarthia and poorly localized low back pain. There was mild vertigo. On examination, the skin showed multiple small scars, some with scabs, a manifestation of recent varicella infection. There were no new lesions. These lesions were mainly on chest wall, abdomen and few on the face. The examination of the nervous system revealed flaccid paraplegia of both lower limbs. There was urinary retention. Plantar responses were absent bilaterally. Deep tendon jerks were absent in the lower limbs but present in upper limbs. Upper limb showed decreased coordination as evidenced by finger nose test. There was mild nystagmus to the left. Dysdiadokokinesia was present on the left side. There was also rebound phenomenon positive. No other cranial nerve abnormality was detected. The patient had marked postural instability. There were no thickened nerves and no vertebral abnormality clinically. Sensory abnormality was ill defined, although the patient reported a tingling sensation down the calf.
Routine blood tests were normal. Vitamin B12 was in normal range. Thyroid functions were normal. Computed tomography (CT) scan of brain and routine X-rays of the vertebra were normal. HIV serology was non-reactive. Cerebrospinal fluid (CSF) examination showed 25 cells/ml; all were lymphocytes and protein was 65 mg/dl. CSF glucose was 70 mg/dl (simultaneous blood glucose 130 mg/dl). The CSF showed increased titer of IgM anti-varicella zoster virus (anti-VZV) antibody (1:256). For CSF, any titer is considered significant. Magnetic resonance imaging (MRI) of brain showed increased contrast uptake and enhancement of tentorium [Figure 1]. Adenosine deaminase [ADA] in CSF was negative. MRI of spinal cord was normal. Serum paired samples at an interval of 10 days showed rising titer of anti-VZV antibody (1:80 to 1:512). The nerve conduction velocity (NCV) studies of both lower limbs showed delayed F-response with ill-persistent F-waves. Based on these findings, a diagnosis of meningitis, cerebellitis and bilateral radiculopathy caused by varicella infection was established.
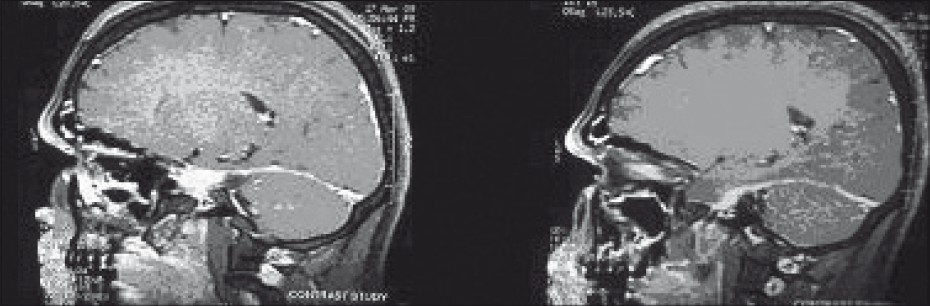
- Enhancement of tentorium is seen in post-contrast T1- weighted imaging
The patient responded to oral acyclovir and prednisolone. The prednisolone was continued for a period of 4 weeks and then tapered. The acyclovir was continued for 10 days. The radiculopathy symptoms were cured earlier but the cerebellar symptoms took 4 months to resolve fully.
Case 2
Polyradiculoneuropathy
A 40-year-old housewife presented to us with sudden onset weakness of all her four limbs, 4 days before admission. The patient had chicken pox in the recent past. On the 10th day, she suddenly realized that she could not move all her four limbs while she was lying down. She could feel someone touching her hands and legs. She had no problems with her memory or mental functions at any point of time.
On general examination, there were hyperpigmented spots all over the body, suggestive of healed chicken pox recently. The general examination otherwise including vitals was found to be within normal limits. Neurological examination revealed normal mentation, no neck stiffness, no cranial nerve affection and no cerebellar signs. The motor examination showed complete flaccid paralysis of all four limbs, power grade 1/5 in all limbs, bilaterally absent deep tendon reflexes and nonresponsive plantar reflexes. Sensory examination showed no involvement with intact bladder and bowel sensations. Gait could not be tested and fundi were normal.
Routine blood investigations were normal. Venereal Disease Research Laboratory test (VDRL) was nonreactive and HIV serology was negative. CT scan brain was also normal. Specific investigations were henceforth carried out and a CSF report showed normal leukocyte count of 5 cells/mm3, all lymphocytes with a markedly raised CSF protein (301 mg/dl) and albumin (64.3 mg/dl), with normal sugar (52 mg/dl). CSF electrophoresis was not done. Acid-fast bacilli (AFB) stain of CSF was negative. NCV study showed diminished C-map of lower limb nerves, suggestive of asymmetric axononeuropathy. Up to 3 mV and 5 mV are the normal values for peroneal nerves and tibial nerves, respectively. Our findings mainly in peroneal nerves were 6.89 and 7.97 mV [Figure 2].

- NCV study showed diminished C-map of lower limb peroneal nerves, suggestive of asymmetric axononeuropathy
A diagnosis of motor polyneuroapthy, demyelinating and axonal, was thus arrived. A serum and CSF VZV titer on day 5 was sent and found to be IgM positive in titer 1:256 and 1:16, respectively. A CSF VZV titer repeated on day 10 showed a rising titer of 1:256 and serum titer was 1:512 on day 15. For CSF, any titer of anti-VZV antibody is considered positive. MRI of spinal cord was unremarkable.
She was on IV methylprednisolone 1 g with supportive therapy in the form of physiotherapy, posture and skin care. The patient was discharged 4 weeks after admission with significant residual weakness, and during follow-up, she is improving very slowly.
Case 3
Acute transverse myelitis
A 37-year-old male patient presented with sudden onset retention of urine and complete weakness of both lower limbs for 1 day prior to admission. He also complained of a belt like sensation around the umbilicus for the last 24–36 hours before admission. Enquiring further, he was found to have a history of high fever 14 days back, followed by appearance of a vesicular rash on the very next day of fever (chicken pox). On examination, he had a fairly normal general examination with normal blood pressure. The neurological examination showed normal higher functions, no signs of meningeal irritation, normal cranial nerves and no evidences of cerebellar involvement. Motor examination revealed normal tone and nearly normal power (4+/5) in both the upper limbs and power of grade 0/5 in both the lower limbs with hypotonia. Jerks were increased in both the lower limbs and plantar was extensor bilaterally. Sensory examination revealed absent sensations to all modalities in chest and below it.
Routine blood and urine examination was normal. Skiagram chest PA view was normal. HIV serology was negative. A CSF study showed slightly raised leukocyte count of 45 cells/mm3; all were lymphocytes. There was mildly elevated protein of 98 mg/dl with normal sugar 56 mg/dl. ADA in CSF was negative (ADA = 3 IU/l; normal <5). An MRI dorsal spine with lumbosacral screening was done which showed hyperintense intramedullary signal changes involving spinal cord from C2 level downward until conus, suggestive of myelitis with cord edema [Figure 3]. For an etiological diagnosis, serum VZV IgM ELISA was done and found to be positive 1:128 on day 1 and 1:512 on day 10 (cut-off: <1:32 negative; 1:32-1:64 equivocal; 1:128 or higher positive). CSF VZV titers were not done because of cost factor.

- MRI dorsal spine T2 sagittal images show hyperintense signals involving dorsal cord
Hence, a definite diagnosis of acute transverse myelitis (ATM) following chicken pox was made. The patient was treated with IV methylprednisolone 1 g daily for 5 days, following which oral prednisolone 1 mg/kg for a total of 2 weeks was given. The weakness improved very slowly. Hence, the patient was discharged with some residual weakness and physiotherapeutic advice. At follow-up, after 1 month, he is doing well and has recovered almost completely although urinary hesitancy persists. No antivirals were used.
Discussion
Common CNS complications of chicken pox are cerebellar ataxia and encephalitis, and rare complications are transverse myelitis, aseptic meningitis, Guillian-Barrι syndrome, meningoencephalitis, ventriculitis, optic neuritis, post-hepatic neuralgia, herpes zoster ophthalmicus, delayed contralateral hemiparesis, peripheral motor neuropathy, cerebral angitis, Reye syndrome and facial paralysis.[2] Complications are generally mild, have a good prognosis and very low mortality.[3] The commonest presentation is encephalitis. In one series by Miller et al, encephalitis accounted for 90% cases and 37% of these had cerebellar involvement.[4]
VZV is a neurotropic human herpesvirus. The pathogenic bases for these complications have been thought to be many. The cause has been postulated as either direct viral invasion[5] or through an immune-mediated allergic mechanism. Most pathologic studies have shown a picture more likely to be allergy-mediated injury.[4] The virus is known to cause vasculitis like episodes and even cerebrovascular accidents have been reported. In immunocompromised host, the virus can invade deeper tissues and the virus has been isolated from brain tissue or ventricles by polymerase chain reaction (PCR).[6] The CSF usually reveals a mononuclear pleocytosis and oligoclonal bands. The oligoclonal IgG is the antibody against VZV. Cerebral angiography also reveals areas of focal arterial stenosis or occlusion. Macroscopically, a predominance of gray–white matter junction lesions is seen. Microscopically, virus is present in affected cerebral arteries,[7] but not in areas of infarction, although in chronic cases virus may be seen in brain parenchyma, usually close to arteries and veins. The primary site of VZV is in cerebral arteries which contain multinucleated giant cells, Cowdry A inclusion bodies, and herpes virus particles. Tentorial and dural enhancement is reported in a few cases of chicken pox.[89] It can be caused by immune-mediated Acute Disseminated Encephalomyelitis [ADEM] or by meningoencephalitis.
Acute cerebellar ataxia is the most common neurological complication of varicella infection, occurring in 1: 4000 children infected with chicken pox. The onset is acute, typically within 1 week of development of rash. But it can occur even up to 3 weeks after the rash onset.[1011]
The disease is rare in adults in endemic countries like India due to early exposure to the virus. But the disease can have serious complications in adults like neurological involvement. Chicken pox is known for rare complications like myelitis, radiculoneuropathy and pure neuropathy. The main treatment for these complications is supportive and steroids have a dubious role.
Radiculopathy is commoner when the virus reactivates, but it can also occur in the primary chicken pox disease.[12] The confirmation of diagnosis is demonstration of the virus antigen or antibody in CSF. But sometimes, these can be negative indicating an immunological basis for the neuropathy. The newer PCR technique is more sensitive.[13] Radiculopathy in isolation after chicken pox is extremely rare and a high degree of clinical suspicion is needed to identify it.[10] The usual treatment is supportive or with steroids. IV Ig can be used in acute demyelinating cases.[14] The disease usually lasts for 2–4 weeks, but can be as short as 3 days or as long as several months.[1516] Persistent cerebellar deficits may develop, albeit rarely, after severe early ataxia.[11] But supportive treatment is mainly important.[17] Table 1 shows signs and symptoms with final diagnosis in outpatients. Table 2 shows major investigation and diagnosis.


Association between Guillain Barre Syndrome [GBS] and chicken pox is rare, and various cases with various pathogenesis mechanisms have been reported since the first case was reported in 1924.[18] Depending on the pattern of involvement (axonal, sensory or autonomic), patients generally improve over several days. Treatment is with IV Ig or plasmapheresis and ventilator support, if the need arises. ATM is a condition of sudden weakness of lower extremities with sensory involvement caused due to inflammation of the spinal cord. Viral disease is responsible for 20-40% cases of ATM such as Epstein-Barr, Rubella, mumps, herpes simplex and VZV.[19] The interval between chicken pox and ATM is variable. According to a report, it can occur with the rash or may be delayed for up to 2 weeks.[20] Treatment of ATM is with corticosteroids, and methylprednisolone intravenous has been found to be effective in one study.[21] Antivirals have a controversial role and physiotherapy has a definitive supportive and rehabilitative role, including in those with bladder involvement.
Conclusion
These three cases show the importance of screening varicella patients for neurological sequelae. These cases often develop after an interval of chicken pox infection, and despite aggressive therapy, they often cause prolonged morbidity.
Source of Support: Nil,
Conflict of Interest: None declared.
References
- The nervous complications of variola, vaccinia and varicella with report of cases. Bull Johns Hopkins Hosp. 1927;40:337.
- [Google Scholar]
- Varicella- zoster virus infection Harrison's Principles of internal medicine. 2008:1103. Chapter 173
- [Google Scholar]
- Severe Chicken- pox Encephalopathy: Treatment with intravenous urea, hypothermia and dexamethasone. Am J Dis Child. 1965;110:137-9.
- [Google Scholar]
- Parainfectious encephalomyelitis and related syndromes: Critical review of neurological complications of certain fevers. Q J Med. 1956;25:427-505.
- [Google Scholar]
- Varicella-Zoster virus infections of the nervous system: Clinical and pathologic correlates. Arch Pathol Lab Med. 2001;125:770-80.
- [Google Scholar]
- Varicella zoster virus, a cause of waxing and waning vasculitis: The New England Journal of Medicine case 5-1995 revisited. Neurology. 1996;47:1441-6.
- [Google Scholar]
- Acute disseminated encephalomyelitis following plasmodium falciparum malaria caused by varicella zoster virus reactivation. Am J Trop Med Hyg. 2005;72:478-80.
- [Google Scholar]
- A case of radiculomyelitis following chicken pox in adulthood. Rinsho Shinkeigaku. 1999;39:817-20.
- [Google Scholar]
- Varicella zoster virus and central nervous system syndromes. Herpes. 2004;11:89A-94A.
- [Google Scholar]
- Sindic Polymerase chain reaction analysis and oligoclonal antibody in the cerebrospinal fluid from 34 patients with varicella-zoster virus infection of the nervous system. J Neurol Neurosurg Psychiatry. 2006;77:938-42.
- [Google Scholar]
- Acute disseminated encephalomyelitis: A case series. Indian Pediatr. 2005;42:367-71.
- [Google Scholar]
- The nervous system diseases associated with varicella. Acute Neurol Scand. 1970;46:279-300.
- [Google Scholar]
- Varicella-Zoster virus: Atypical presentations and unusual complications. J Infect Dis. 2002;186:91-8.
- [Google Scholar]
- Neurological complications of varicella-zoster virus (VZV) infection. No To Hattatsu. 1993;25:128-34.
- [Google Scholar]
- Zur pathologis chen anatomie des nerven systems beim herpez zoster. Z Gesamate Neurol Psychiatr. 1924;89:171.
- [Google Scholar]
- Post varicella acute transverse myelitis in a previously vaccinated child. Paedaitr Neurol. 2008;38:370-1.
- [Google Scholar]
- High dose methylprednisolone in severe acute myelopathy. Arch Dis Child. 1997;76:167-8.
- [Google Scholar]






