Translate this page into:
Mild traumatic brain injuries in adults
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Mild traumatic brain injury (mTBI) is the commonest form of TBI. Though the name implies, it may not be mild in certain cases. There is a lot of heterogeneity in nomenclature, classification, evaluation and outcome of mTBI. We have reviewed the relevant articles on mTBI in adults, particularly its definition, evaluation and outcome, published in the last decade. The aspects of mTBI like pediatric age group, sports concussion, and postconcussion syndrome were not reviewed. There is general agreement that Glasgow coma score (GCS) of 13 should not be considered as mTBI as the risk of intracranial lesion is higher than in patients with GCS 14–15. All patients with GCS of <15 should be evaluated with a computed tomography (CT) scan. Patients with GCS 15 and risk factors or neurological symptoms should also be evaluated with CT scan. The outcome of mTBI depends on the combination of preinjury, injury and postinjury factors. Overall outcome of mTBI is good with mortality around 0.1% and disability around 10%.
Keywords
Concussion
Glasgow coma score
head injury
mild traumatic brain injury
Introduction
No head injury is too trivial to be ignored.
-Hippocrates
Head injury, now better known as traumatic brain injury (TBI), is a silent epidemic of post industrialization era. It affects young and productive people, leading to significant loss of life and economy. Among TBI, the commonest form is mild traumatic brain injury (mTBI). Though the name suggests the benign nature of condition in terms of risk to life, however, the consequences of mTBI can impair general health and functioning. There is lot of variation in the management of mTBI globally. We have reviewed the articles published on mTBI during the last decade using search items “mild head injury”, “mild traumatic brain injury”, “minor head injury”, and “minor traumatic brain injury”. The articles dealing with definition, classification and evaluation of mTBI in adults were selected. The aspects of mTBI like pediatric age group, sports concussion, and postconcussion syndrome were not reviewed. Articles which had substantial information as to influence practical management, as compared to older articles, were considered relevant. This brief review is based on these articles.
Definition
How mild is mTBI? The answers to this question are many, and probably all of them are correct. Head injury is defined by World Health Organization (WHO) as “any injury to scalp, skull, or brain with ICD10 codes S00.0 to S09.9”. There is no sub-classification regarding severity of injury though there is a mention of concussion (commotio cerebri) coded as S06.0 under the heading of intracranial injury.[1] The presence of concussion indicates TBI and the absence of it indicates head injury. Concussion can vary in severity, but is commonly perceived as a brief and reversible phenomenon, which lay people relate to as mild head injury.
The definition of mTBI has been given by several authorities depending on their health policies, and keeping the need for evaluation in the background. The key components to definition of mTBI are Glasgow coma score (GCS), duration of loss of consciousness (LOC), and post traumatic amnesia (PTA). Conventionally, the GCS has been used to classify the severity of TBI. GCS 13–15 is considered as mTBI. This simple definition is very convenient for hospital-based epidemiological studies. However, the large spectrum of clinical and radiological findings in patients with GCS 13–15 leads to poor specificity of this definition. Hence, GCS was further split into 13 versus 14–15, with GCS 13 having a higher probability of an abnormal computed tomography (CT) scan.[23] Whether patients in GCS 13 should be reclassified and put in the moderate injury group needs to be further studied.
The duration of LOC and PTA is an index of severity of injury. As far as LOC is concerned, the basis of cut-off duration (30 minutes) is not clear. It is also not clear as to beyond what time duration after mTBI, PTA should be considered significant. It has been observed that even if PTA is up to 24 hours, patients can be expected to have a good outcome. However, a retrograde amnesia beyond 30 minutes is always considered as abnormal.[4] We feel that assessment of PTA is not always accurate and should not be a strict criterion for classification of mTBI.
Centre for Disease Control (CDC) has defined mTBI as follows.[5].
Any period of observed or self-reported
-
Transient confusion, disorientation, or impaired consciousness;
-
Dysfunction of memory around the time of injury; and
-
LOC lasting less than 30 minutes.
Observed signs of neurological or neuropsychological dysfunction are
-
Seizures acutely following injury to the head;
-
Irritability, lethargy, or vomiting following head injury; and
-
Headache, dizziness, irritability, fatigue, or poor concentration.
The Neurotraumatology Committee of the World Federation of Neurosurgical Societies (WFNS) proposed to use the terminology mTBI to encompass all the categories of injuries which were previously called as “minor” or “trivial”. The mTBI is further classified into low, medium, and high risks based on GCS and the presence of neurological symptoms.[3] The neurological symptoms included are LOC, PTA, vomiting and diffuse headache. Patients with GCS 15 without any neurological symptoms are classified as “low-risk”, and those with any of the above symptoms are classified as “medium-risk“. Patients with GCS 14 or15 with a skull fracture or neurological deficits are classified as “high-risk”. Presence of any of the following risk factors, i.e., coagulopathy, drug or alcohol consumption, previous neurosurgical procedures, pretrauma epilepsy, or age more than 60 years, also indicates high-risk irrespective of admission GCS.[3]
European Federation of Neurosurgeons (EFNS) has defined and classified mTBI based on the parameters GCS, PTA or LOC, and risk factors into four categories.[6] The risk factors include unclear or ambiguous accident history, continued post-traumatic amnesia, retrograde amnesia longer than 30 minutes, trauma above the clavicles including clinical signs of skull fracture, severe headache, vomiting, focal neurological deficit, seizure, age less than 2 or more than 60 years, coagulation disorders, high-energy accident [according to Advanced Trauma Life Support (ATLS) principles, a high-energy vehicle accident is defined as initial speed >64 km/hour, major auto-deformity, intrusion into passenger compartment >30 cm, extrication time from vehicle >20 minutes, falls >6 m, roll over, auto-pedestrian accidents, or motor cycle crash >32 km/hour or with separation of rider and bike], and intoxication with alcohol/drugs. Patients with GCS 15 without LOC, PTA or risk factors are classified as category 0, GCS 15 with LOC or PTA as category 1, GCS 15 with risk factors as category 2, and GCS 13–14 as category 3.
The most comprehensive definition is the one given by WHO Collaborating Centre Task Force on mTBI.[7] They have expanded the previous definition of mTBI as “an acute brain injury resulting from mechanical energy to the head from external physical forces”. Operational criteria for clinical identification include:
-
One or more of the following: confusion or disorientation, loss of consciousness for 30 minutes or less, post-traumatic amnesia for less than 24 hours and/or other transient neurological abnormalities such as focal signs, seizure, and intracranial lesion not requiring surgery.
-
GCS of 13–15, 30 minutes post-injury or later upon presentation for healthcare.
These manifestations must not be due to drugs, alcohol, medications, caused by other injuries or treatment for other injuries (e.g., systematic injuries, facial injuries or intubation), caused by other problems (e.g., psychological trauma, language barrier or coexisting medical conditions) or by penetrating craniocerebral injury. This definition highlights the complex nature of mTBI, including numerous factors which potentially complicate initial diagnosis and treatment.
We feel that any TBI precluding return to work on the same day should not be considered minor.
Epidemiology
The annual global incidence rates of TBI range from 91 per 100,000 population to 546 per 100,000. The mTBI constitutes 70–90% of all head injuries, with rates of hospital treatment for mTBI ranging from 100 to 300 per 100,000 population per annum. This high variability in incidence is due to sampling of population ranging from only hospitalized patients to all the patients who visit emergency department and also due to lack of uniform definition.[1] The actual incidence of mTBI is difficult to estimate as these patients are managed by various specialists at different times from the accident. Many patients do not come to trauma center and are not seen by neurosurgeons. A large number of cases are not treated at hospitals; the actual rate is possibly in excess of 600 per 100,000 cases.[8] There is bimodal distribution of mTBI, with peaks at age group 15–24 years and after 65 years.[9]
Evaluation
CT scan is the best tool to evaluate patients with mTBI in the acute phase. There is no role of X-ray skull, and magnetic resonance imaging (MRI) is limited to research and not recommended for management at present. Various authorities have listed indications for CT scan [Table 1]. The guidelines should detect the entire patients at risk of intracranial injury with a minimum number of scans required to detect one positive case.[34610–14] Four or more of these guidelines agreed on the following indications for CT scan:
-
GCS < 15
-
GCS 15 with any of the following symptoms or signs:
-
Vomiting
-
LOC
-
Age > 60 years
-
Focal neurological deficits
-
Skull fracture
-
Posttraumatic seizures
-
PTA
-
Headache
-
Coagulopathy
-
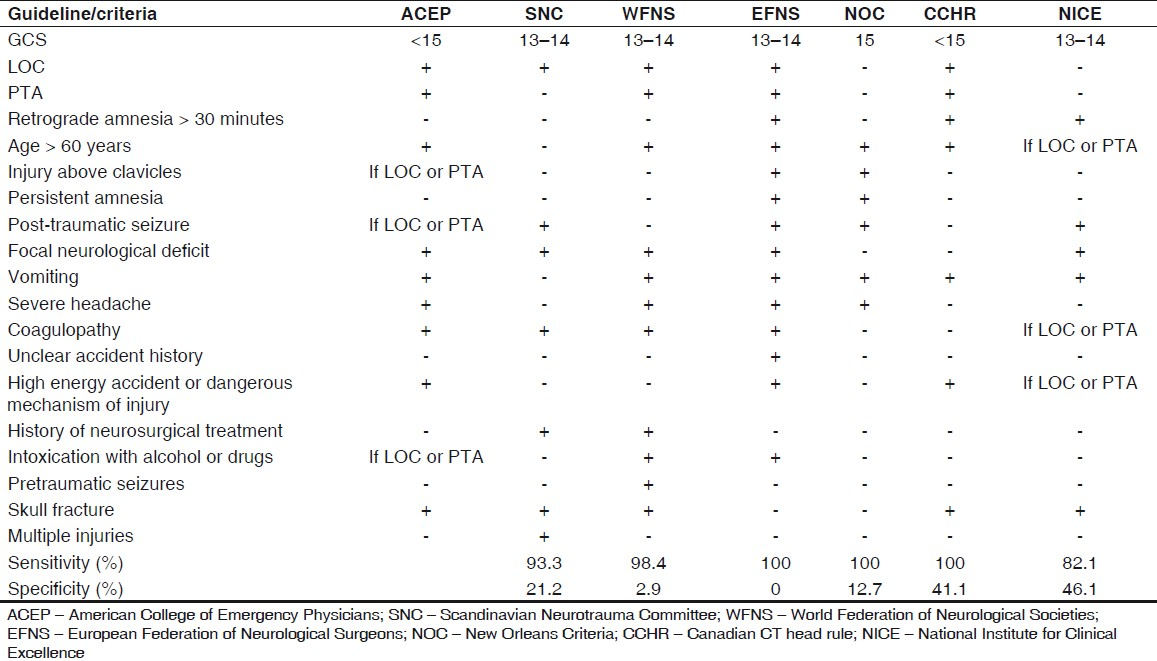
The initial CT scan of head is abnormal in approximately 8% cases and depicts lesion requiring neurosurgical intervention in 0.9%. The CT scan findings in mTBI in decreasing order are: subgaleal hematoma, skull fracture, brain swelling, subarachnoid hemorrhage, subdural hematoma, contusion, and extradural hematoma.[15–17]
There are no guidelines regarding timing of CT scan after mTBI. If a scan is done too early, it may be normal or show insignificant findings. In such cases, there is a role of delayed or repeat CT scan. In a study of 898 patients with mTBI, in which all patients underwent CT scan, 83 (9%) patients showed increase in size of previous lesion or appearance of new lesions. The timing of repeat CT scan ranged from less than 24 hours to 7 days. The commonest new lesion was hygroma, followed by delayed traumatic intracerebral hematoma and delayed epidural hematoma. The former was seen in patients with headache, and the later two in patients who had neurological deterioration. The lesions which required urgent surgery were detected in the first 3 days. Repeat CT scan must be done in the case of intracranial hematomas and contusions if the patient is not operated upon. Repeat CT scans should also be done for patients with clinical deterioration or with new clinical symptoms, even if the initial CT scan was normal.[2]
At our institute, we recommend a CT scan of head for all the patients who are drowsy and disoriented (GCS 13) at presentation, and who remain drowsy or disoriented (GCS 14) even after 6 hours of injury. During this time, the patient is monitored closely and an immediate scan is done if there is any sign of deterioration. In addition to above listed indication, we also ask for a CT scan for a patient who does not have a care taker at home and at times on insistence of patients who like to be reassured. A repeat CT scan is done within 12–24 hours of initial CT scan with positive findings. A plain X-ray skull and MRI is never asked for mTBI at our institute.
Treatment
The acute treatment of the patients with mTBI is symptomatic. They should be observed for any clinical signs of deterioration. There is no consensus regarding the duration of observation. The possibility of deterioration is maximum during the first 24 hours after injury. The other option is getting an early CT scan. Clinical outcomes of the patients are similar to those in patients admitted for observation or early CT scans.[18] However, the cost of observation is more than that of getting a scan done and discharge if no significant abnormalities are seen. The cost of patient care is lowered to one third if a CT scan is asked for.[19]
After discharge, most of the patients do not require further treatment. About 10–15% of patients continue to see their physicians for various symptoms. The evidence base for management of these symptoms is very limited. Provision of information early after injury results in reduced symptom reporting. The influence of factors not directly related to the brain injury may play an important role for formulating more uniform management guidelines.[20] In a systematic review of psychological therapy after mTBI, positive outcomes of cognitive rehabilitation programs were found. There was no difference between minimal versus intensive patient education programs. Cognitive behavioral therapy (CBT) with an educational component was found to be superior to education-based supportive counseling.[16]
Educational information about mTBI symptoms, including reassurance that these are likely to recover, or early screening assessment and management advice with encouragement of gradual return to usual activities, should be done. Encouragement of hospitalized mTBI patients to get up from bed early, provision of physiotherapy and educational information, physician follow-up and encouragement to resume normal activities results in fewer days off work. The symptoms directly associated with mild head injury from those due to other factors such as pain, stress, personality issues or litigation, which may contribute to ongoing disability, should be differentiated and addressed.[21]
The role of pharmacotherapy in such patients is limited. In a systematic review of treatment for mTBI, the effect of desmopressin acetate versus placebo on mental performance, information processing rate and immediate recall was found to be significant. The result of amitriptyline or sertraline on a variety of outcomes is inconclusive. Dihydroergotamine was found to have positive results on memory, sleep, and dizziness.[22] Citicoline which is used for stroke has also been tried in mTBI. Compared to placebo, 1 g/day of citicoline for 1 month in patients with mild head injury did not reduce either the number of working days lost or the postconcussion symptoms (PCS). There was no difference in the quality of life between patients taking citicoline and placebo.[23]
Outcome
Prediction
The predictors of outcome following mTBI can be classified in three groups: preinjury, injury and post-injury [Table 2].[24] Poor outcome is due to combinations of these factors.

Preinjury factors
Age
Increasing age is associated with poor outcome. The cut-off of 60 or 65 years age has been shown in some studies.
Sex
The most controversial predictor of outcome after TBI is sex. In a study comprising 1425 patients when the outcome was assessed 3 months after mTBI, males had significantly lower odds of being in a higher PCS score category [odds ratio (OR): 0.62; 95% confidence interval (CI): 0.50, 0.78]; this association appeared to be more prominent during child-bearing years for females. Males and females did not significantly differ with respect to the odds of poorer outcome as defined by the number of days to return of normal activities or the number of days of work missed. Female sex was associated with significantly higher odds of poor outcome after mTBI, as measured by PCS score, after control for appropriate confounders.[24]
Socioeconomic status
Compared to patients in high-income countries, patients in low- and middle-income countries have same odds of mortality but have half the odds of disability following mTBI. Sociocultural and environmental factors may explain the lower levels of disability after mTBI.[25]
Genetic
APOE e4 genotype is associated with poor survival following severe head injury. In two studies on mTBI, the presence of APOE e4 allele did not show significant negative effect on the outcome after mTBI for GOS and neuropsychological measures.[26] Besides affecting global functional and neuropsychological outcome, APO E status also influences hormonal deficiency after mTBI. The APO E3/E3 genotype decreases the risk of hypopituitarism after TBI.[27]
Injury factors
Additional injuries
Though the patients with additional injuries are in a process of recovery even 6 months after injury, they do not report more severe postconcussional than the patients with isolated mTBI.[28]
CT scan findings
In the follow-up of CHIP study, the patients with diffuse axonal injury, intraparenchymal lesions, and subdural hematoma had significantly poorer GOS outcome. The presence of EDH and basal skull fracture was associated with more severe PCS. There was no relationship with outcome in patients with traumatic SAH, and isolated linear or depressed fracture.[2930] Other studies also found positive correlation of outcome with CT scan findings.[3132] Contrary to CHIP study, Radbound University Brain Injury Cohort Study (RUBICS) concluded that clinical features are stronger predictors of outcome than CT scan findings as measured on Glasgow Outcome Scale extended (GOSE). They found that the predictive value of scheme base injury severity score (ISS), alcohol intoxication on the day of injury and age were significant predictors.[33]
Clinical features
Though LOC is a key criterion for PCS diagnosis, it is not predictive of sustained PCS.[34]
Post-injury factors
Symptomatic patients, who believe that their symptoms have serious negative consequences on their lives and will continue to do so, are at heightened risk of experiencing significant enduring PCS. Whatever other physical or psychological factors may be involved, patients’ perceptions of their illness early after head injury play a part in the persistence of PCS.[35]
Assessment
Measurement of outcome based solely on the GOS is not appropriate, and it is recommended that a differentiated outcome scale involving emotional, behavioral, cognitive, and physical domains should be used. Outcomes of interest after mTBI include: improvement in functioning and quality of life, measures of activity and participation, and measures of neuropsychological functioning and psychosocial adjustment.[21] GOSE may be used to measure outcome after mTBI and the outcome should be dichotomized into favorable outcome as good recovery (GOSE 8) versus unfavorable for rest of the scores (GOSE 1–7).[2]
The outcome after mTBI is generally good. The mean mortality of patients is very low (0.1%).[16] Nearly 10% of patients with mTBI need continuous and long-term supportive care.[36] Quality of life studies after 3–12 months of mTBI have shown that health and functioning domain is affected in 10–80%, headache being the commonest symptom. Psychological and spiritual domain is affected in 12–30%, commonest cause of which is depression. Social and economic domain is affected in 12-15% as reflected by non-return to work.[37]
Endocrine Function after Mild Traumatic Brain Injury
The prevalence of endocrine dysfunction after TBI varies depending on the methods used to assay hormone levels, definitions of specific hormone deficiency and timing of evaluation after injury. The prevalence of growth hormone (GH) deficiency is 2–39%, adrenal insufficiency 0–60%, hypothyroidism 0–19%, and hypogonadism 0–29%.[38] Endocrine dysfunction after TBI is believed to occur only in severe cases. However, this may not be true as even in mTBI pituitary hormones deficiency can occur. The prevalence of hypopituitarism after mTBI is 16.8% as compared 10.9% in moderate and 35.3% in severe TBI.[39] In mTBI, isolated pituitary hormone deficiencies are more common compared to multiple pituitary hormone deficiencies in severe TBI. GH deficiency is the commonest (29%), followed by the deficiency of adrenocorticotrophic hormone (ACTH) (9.6%) and gonadotropins (3.2%).[40] Hormonal assessment should be done for patients of mTBI who need hospitalization for at least 24 hours, who have an abnormal intial CT and who have signs and symptoms of pituitary dysfunction at any time after the event. Patient should be evaluated with dynamic tests for ACTH and GH at 3, 6, and 12 months after mTBI. The clinical features of hormone deficiency are quite vague and indistinguishable from PCS. The relative contribution of hormone deficiency on quality of life after mTBI is still not known and whether hormone replacement will improve the outcome needs to be investigated further.
Conclusion
mTBI is common, with a significant impact on public health and healthcare cost. Proper understanding, evaluation, and prompt management would go a long way in improving the quality of life of the victim of mTBI. As mTBI may not be so mild in a few cases, its prevention should be encouraged by the use of helmets for motorcyclists and bicyclists.
Source of Support: Nil,
Conflict of Interest: None declared.
References
- Head Injury: A Multidisciplinary Approach. In: Whitfield PC, Thomas EO, Summers F, Whyte M, Hutchinson PJ, eds. Head Injury: A Multidisciplinary Approach. Cambridge: Cambridge University Press; 2009. p. :1-10.
- [Google Scholar]
- A more detailed classification of mild head injury in adults and treatment guidelines. J Korean Neurosurg Soc. 2009;46:451-8.
- [Google Scholar]
- Neurotraumatology Committee of the World Federation of Neurosurgical Societies.Defining acute mild head injury in adults: a proposal based on prognostic factors, diagnosis, and management. J Neurotrauma. 2001;18:657-64.
- [Google Scholar]
- Reliability of clinical guidelines in the detection of patients at risk following mild head injury: Results of a prospective study. J Neurousrg. 2004;100:825-34.
- [Google Scholar]
- Centers for Disease Control and Prevention. 2005. Available from: http://www.cdc.gov/ncipc/pub-res/tbi_ toolkit/toolkit.htm/
- [Google Scholar]
- EFNS guideline on mild traumatic brain injury: Report of an EFNS task force. Eur J Neurol. 2002;9:207-19.
- [Google Scholar]
- WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. Methodological issues and research recommendations for mild traumatic brain injury: The WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004;43:113-25.
- [Google Scholar]
- Incidence, risk factors and prevention of mild traumatic brain injury: Results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004;43:28-60.
- [Google Scholar]
- Head Trauma: Basic, Preclinical, and Clinical Directions. In: Miller L, Ayes R, eds. Head Trauma: Basic, Preclinical, and Clinical Directions. New York: John Wiley and Sons; 2001. p. :327-47.
- [Google Scholar]
- Minor head injury: guidelines for the use of CT--a multicenter validation study. Radiology. 2007;245:831-8.
- [Google Scholar]
- Clinical policy: Neuroimaging and decision making in adult mild traumatic brain injury in the acute setting. Ann Emerg Med. 2008;52:714-48.
- [Google Scholar]
- Scandinavian guidelines for initial management of minimal, mild, and moderate head injuries: The Scandinavian Neurotrauma Committee. J Trauma. 2000;48:760-6.
- [Google Scholar]
- External validation of the Canadian CT Head Rule and the New Orleans Criteria for CT scanning in patients with minor head injury. JAMA. 2005;294:1519-25.
- [Google Scholar]
- Comparison of the Canadian CT head rule and the New Orleans criteria in patients with minor head injury. JAMA. 2005;294:1511-8.
- [Google Scholar]
- The changing face of mild head injury: Temporal trends and patterns in adolescents and adults from 1997 to 2008. Injury. 2010;41:968-72.
- [Google Scholar]
- Mild head injury – mortality and complication rate: Meta-analysis of findings in a systematic literature review. Acta Neurochir. 2003;145:843-50.
- [Google Scholar]
- CT scan findings in mild head trauma: A series of 2,000 patients. Arq Neuropsiquiatr. 2002;60:204-10.
- [Google Scholar]
- for the OCTOPUS Study Investigators Medical outcome after immediate computed tomography or admission for observation in patients with mild head injury: Randomised controlled trial. BMJ. 2006;333:465.
- [Google Scholar]
- Mild head injury: Observation or computed tomography. Economic aspects by literature review and decision analysis? Emerg Med J. 2004;21:54-8.
- [Google Scholar]
- Rehabilitation interventions after mild head injury. Curr Opin Neurol. 2005;18:692-7.
- [Google Scholar]
- A systematic review of psychological treatments for mild traumatic brain injury: An update on the evidence. J Clin Exp Neuropsychol. 2009;31:20-38.
- [Google Scholar]
- A systematic review of treatments for mild traumatic brain injury. Brain Inj. 2005;19:863-80.
- [Google Scholar]
- Role of citicoline in the management of mild head injury. Indian J Neurotrauma. 2009;6:49-52.
- [Google Scholar]
- Sex differences in outcome after mild traumatic brain injury. J Neurotrauma. 2010;27:527-39.
- [Google Scholar]
- Patient outcome after traumatic brain injury in high- middle- and low-income countries. Int J Epidemiol. 2009;38:452-8.
- [Google Scholar]
- Apolipoprotein E polymorphism and outcome after mild to moderate traumatic brain injury: A study of patient population in India. Neurol India. 2010;58:264-9.
- [Google Scholar]
- Apolipoprotein E3/E3 genotype decreases the risk of pituitary dysfunction after traumatic brain injury due to various causes: Preliminary data. J Neurotrauma. 2008;25:1071-7.
- [Google Scholar]
- Impact of additional extracranial injuries on outcome after mild traumatic brain injury. J Neurotrauma. 2006;23:1561-9.
- [Google Scholar]
- Predicting intracranial traumatic findings on computed tomography in patients with minor head injury: The CHIP prediction rule. Ann Intern Med. 2007;146:397-405.
- [Google Scholar]
- Outcome after complicated minor head injury. AJNR Am J Neuroradiol. 2008;29:506-13.
- [Google Scholar]
- Patients with mild traumatic brain injury: Immediate and long-term outcome compared to intra-cranial injuries on CT scan. Brain Inj. 2006;20:1131-7.
- [Google Scholar]
- Outcome after mild to moderate blunt head injury: Effects of focal lesions and diffuse axonal injury. Brain Inj. 2001;15:401-12.
- [Google Scholar]
- Outcome Prediction in Mild Traumatic Brain Injury: Age and Clinical Variables Are Stronger Predictors than CT Abnormalities. J Neurotrauma. 2010;27:655-68.
- [Google Scholar]
- Does brief loss of consciousness affect cognitive functioning after mild head injury? Arch Clin Neuropsychol. 2000;15:643-8.
- [Google Scholar]
- Illness perceptions and outcome in mild head injury: A longitudinal study. J Neurol Neurosurg Psychiatry. 2007;78:644-6.
- [Google Scholar]
- Epidemiology of traumatic brain injuries: Indian scenario. Neurol Res. 2002;24:24-8.
- [Google Scholar]
- Mild traumatic brain injury: Determinants and subsequent quality of life: A review of the literature. J Neurosci Nurs. 2007;39:260-72.
- [Google Scholar]
- Hypopituitarism following traumatic brain injury: Prevalence is affected by the use of different dynamic tests and different normal values. Eur J Endocrinol. 2010;162:11-8.
- [Google Scholar]
- Hypothalamopituitary dysfunction following traumatic brain injury and aneurysmal subarachnoid hemorrhage: A systematic review. JAMA. 2007;298:1429-38.
- [Google Scholar]
- Pituitary function in subjects with mild traumatic brain injury: A review of literature and proposal of a screening strategy. Pituitary. 2010;13:146-53.
- [Google Scholar]






