Translate this page into:
Zidovudine-induced myopathy: A study in Indian patients
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Context:
Literature is replete with studies on zidovudine-induced myopathy after prolonged use (use beyond 270 days on an average). However, all these studies have been done on patients of Caucasian, American and African ethnic origin. No such study has been carried out in Indian patients to our knowledge.
Aims:
To determine the correlation of zidovudine usage with serum creatine phosphokinase (CK) levels, clinical muscular weakness and muscle histology in Indian patients, we studied 147 physically active, Human Immunodeficiency Virus infected men on prolonged zidovudine-based antiretroviral therapy (ART).
Settings and Design:
Cross-sectional study on hospital follow-up patients of HIV infection.
Materials and Methods:
All cases on ART who reported to our canter during a period of 18 months were evaluated for symptoms (muscle fatigue, myalgia), objective muscle strength (testing clinically) and serum CK levels, and a select group was evaluated by muscle biopsy. These patients were on zidovudine for 1 to 7 years.
Results:
None of the patients studied had significant symptoms or objective muscle weakness and only a small fraction (10.8% of cases) had marginally raised serum CK levels. All muscle biopsies were normal on light microscopy.
Conclusions:
Zidovudine myopathy may be a constraint for use of the drug in the western population; however, it is a well-tolerated drug as regards myopathy in our study on Indian patients.
Keywords
Myopathy
zidovudine
antiretroviral therapy
Introduction
Myopathy can present across the spectrum of Human Immune Deficiency Virus (HIV) infection: during the syndrome of seroconversion, as an initial symptom of AIDS or as an adverse effect of antiretroviral therapy (ART). Zidovudine, which belongs to the nucleoside analogue group of ART drugs, has been marked with a black box warning for prolonged use due to its association with symptomatic myopathy. All nucleoside analogue drugs are known to induce mitochondrial dysfunction due to their varying affinity for mitochondrial gamma DNA polymerase. This affinity results in interference with mitochondrial replication, resulting in mitochondrial DNA depletion and dysfunction.[1] The relative potency of the nucleosides in inhibiting mitochondrial gamma DNA polymerase in vitro is the highest for zalcitabine, followed by didanosine, stavudine, lamivudine, zidovudine and abacavir.[2]
Toxicity has been reported in patients receiving long-term treatment and generally resolves with discontinuation of the offending drug. A possible genetic susceptibility to these toxicities has also been suggested.[12]
Nausea, vomiting, abdominal discomfort, headache, myalgia and dizziness are the common side effects of zidovudine. The drug has been shown to have a good nervous system penetration without neurotoxicity. Elevations in lactate dehydrogenase (LDH), creatine phosphokinase (CK) and transaminases are known to be the common biochemical abnormalities with zidovudine use.[3] Zidovudine-related myopathy is not very common and cardiomyopathy is also rare. Zidovudine-induced myopathy is characterized by reversible muscle weakness, wasting, myalgia, fatigue and elevated CK levels. Some zidovudine-treated patients with normal muscle strength experience excessive fatigue, myalgia, or transient mild CK elevations that improve when zidovudine is stopped.[4] Other toxicities of zidovudine include macrocytic anemia (red blood cell mean corpuscular volume almost always elevated), and rarely, neutropenia and myelotoxicity with other myelosuppresive drugs like ganciclovir, cotrimoxazole, dapsone, etoposide, pyrimethamine, interferon, duanorubicin, vinblastine, vincristine, sulfadiazine, amphotericin B and ribavirin.[3]
All these studies have been carried out on patients of Caucasian, American and African ethnic origin. No such study has been carried out in Indian patients to our knowledge. With an aim to determine the effect of zidovudine usage on the development of myopathy in Indian patients, we decided to study 147 physically active, Human Immunodeficiency Virus infected men on prolonged zidovudine based ART.
Materials and Methods
We evaluated 147 HIV positive patients on ART who were on follow-up at our centre. All HIV cases on ART who reported to our center for a period of 18 months were evaluated.
Inclusion criteria
All patients on zidovudine (600 mg/day in two divided doses) based ART, for a period of more than 1 year, were selected (range: 1 year to a maximum of 7 years). All these patients were active individuals leading an apparently normal life at their place of work and home.
Exclusion criteria
Any HIV positive individual admitted for any debilitating illness, terminal cases and illness producing a bed bound state or extreme weakness was excluded.
All the patients were evaluated for symptoms of excessive fatigue, muscular pain and reduced endurance. Symptoms at less than 2 km walk on a plain ground or at less than 30 minutes as time duration were considered by us as a satisfactory cut off. Muscle strength was examined clinically for all cases. The muscle strength was assessed using the Medical Research Council (MRC) scale for muscle strength. Grade 5 on the scale was taken as normal and any grade of 4 or below was taken as weakness. All these patients were evaluated for CK levels during their routine follow-ups. We took a cut off level of above 174 IU/L to be significant. The upper limit of normal for CK levels in our laboratory was 174 IU/L. Twenty patients with either the clinical symptoms as above or raised CK levels or both were subjected to muscle biopsy from the quadriceps, after obtaining due consent. The muscle biopsies were subjected to light microscopy. Electron microscopy (EM) and enzyme histochemistry were not done as facilities for the same were not available at our center.
Results
None of the patients themselves complained of muscle pain, fatigue or reduced endurance as a presenting symptom. However, on direct questioning, 34 patients out of 147 (23.1%) admitted to having muscle fatigue for more than ordinary activities which normal individuals could perform on a day-to-day basis without symptoms. These symptoms were on activities more than our cut off criteria for physical symptoms. All subjects had normal muscle strength.
Sixteen out of 147 patients (10.8%) on zidovudine had raised CK levels. The upper limit of CK in our laboratory is 174 IU/L. The range of CK in these patients was 178-527 IU/L, with a mean of 227 IU/L.
Twenty-five patients had only symptoms of muscle fatigue but normal CK levels, 7 patients had asymptomatic raised CK levels but no symptoms and nine patients had both [Figure 1]. Thus, a total of 41 patients out of 147 patients (27.8%) studied had either raised CK levels or symptoms of muscle fatigue or both and 106 patients scored nil for symptoms and raised CK levels.
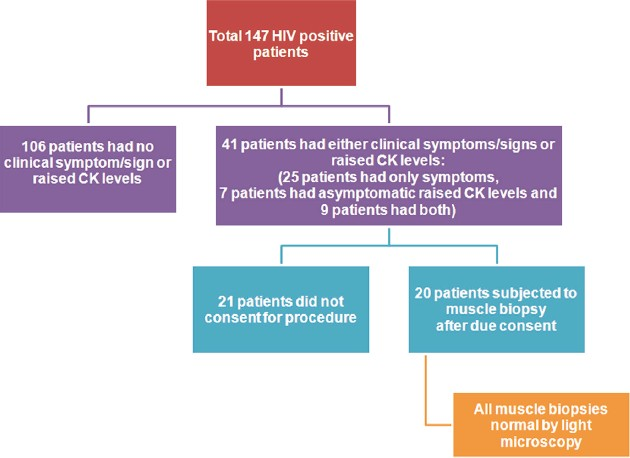
- Diagrammatic break-up of the study group
Out of the 41 patients with raised CK levels or symptoms as above, muscle biopsy was carried out in 20 patients. Others either did not give their consent or had other pressing domestic/service reasons for an early disposal. Muscle biopsies were analyzed by light microscopy and were normal in all cases [Figures 2 and 3].

- Muscle biopsy : normal study ( H and E, ×10)

- Muscle biopsy : normal study (H and E, ×40)
The time range of zidovudine treatment in the 41 clinical/biochemically “positive” group was from 3 years 2 months to 6 years 4 months, with a mean of 3 years 8 months (mean intake of 792 g per head). The mean time duration of zidovudine treatment for all the 147 cases was 3 years 10 months (mean intake of 828 g per head). The mean duration of treatment in the nine cases that had both symptoms and raised CK levels was 4 years 1 month (mean intake of 882 g per head).
Discussion
Skeletal muscle involvement may occur at all stages of HIV infection and represents the first manifestation of the disease in some patients. The myopathy in HIV-infected patients can be subdivided into (a) HIV-associated myopathy, a myopathy that meets the criteria for polymyositis in a majority of patients, and in some for acquired nemaline myopathy; (b) zidovudine myopathy; (c) the HIV-wasting syndrome and other AIDS-associated cachexia; (d) opportunistic infections and tumor infiltration of skeletal muscle; (e) vasculitic processes and iron pigment deposits; (f) HIV-associated myasthenia gravis and (g) rhabdomyolysis.[45]
Zidovudine (ZDV), a DNA chain terminator, results in overt myopathy when a critical threshold of molecular, histological, and biochemical dysfunction of mitochondria is crossed, which seems to vary between individuals.[4] The myopathy described is with ragged-red fibers and mitochondrial DNA depletion in muscle.[6] The myopathy has been described as reversible with clinical complaints disappearing in about 3 months after drug cessation.[7]
Many western studies have mentioned that the clinical effectiveness of ZDV is constrained due to its association with increased adverse effects such as myopathy.[1] In a study by Peters et al., clinical and biochemical evidence of proximal myopathy was seen in 7 of 88 patients (7.9%) who had been receiving zidovudine. Out of these 88 patients, 41 had received zidovudine for more than 270 days, and all the 7 patients with myopathy belonged to this subgroup (17%) and no such finding was present in those on a lesser duration of therapy or in controls. In the same study, only four out of these seven patients had histological evidence of myopathy on light microscopy.[8] CK values of above 300 IU/L were taken into consideration in many of these studies.[9] Various studies have a higher incidence of myopathy ranging from 8 to 50% based on clinical, biochemical or histopathological criteria.[48] Some studies have correlated the occurrence of myopathy with a higher dosage of zidovudine, whereas other studies found no relation.[49]
Whereas the biochemical levels of CK taken into consideration in other studies have been above 300 IU/L, we took a limit of 174 IU/L as cut off, and found 16 patients out of 147 or 10.8% of total subjects studied with “raised” CK levels. If we raise the bar to 300 IU/L as in other studies, then we have only 2 cases who had high CK levels or just 1.36% of total patients.
Our study revealed that actually none of the patients had brought out the symptoms of muscle fatigue or reduced endurance on their own as a presenting complaint. The ones who experienced symptoms were at levels of more than ordinary activity and thus not affecting the average quality of life. In overview, the usage of zidovudine in these patients for duration of 1–7 years also was not associated with any clinically significant symptoms.
If we analyze broadly the clinically “symptomatic” group (which in practical clinical sense should be nil) and those with CK above 300 IU/L, then the fraction of patients suspected to have myopathy comes down even further.
A muscle biopsy by light microscopy in zidovudine myopathy typically shows ragged-red fibers, fiber-size variation with atrophic, necrotic and degenerating fibers of varying severity culminating in necrosis, lipid droplets and lymphoplasmocytic inflammatory response or absence of inflammation. In our study, all 20 muscle biopsies were normal by light microscopy. It is possible that if they were subjected to enzyme histochemistry and EM, some may have shown changes compatible with myopathy. However, because of the cost involved and lack of infrastructure, the tissues were not processed further.
Thus, we saw a relative paucity of symptoms of muscle fatigue or loss of endurance in our patients. The ones who admitted to having the same did not find them bad enough to be reported as a presenting complaint which indirectly indicates the mild nature of the problem. None of the 147 cases had objective evidence of muscle weakness. The muscle biopsies were also normal as observed by light microscopy which is a good screening procedure in general for detection of zidovudine myopathy. Thus, none of our patients appeared to have myopathy. If EM studies were to be conducted, then possibly some changes may have been detected. The authors feel that since overall, all these patients have been on the drug (zidovudine) for periods ranging from 1 to 7 years with no substantial clinical, biochemical or histological facts to substantiate myopathy, the drug is probably well tolerated in these patients.
The variance in our study could be postulated to be due to a generally good diet and nutrition in our subjects as they belonged to a comfortable socioeconomic background (with an average income of $5000 per annum), a rigid follow-up procedure for the patients, availability and good compliance of free ART and better general hygiene and sanitation with access to safe and hygienic food and water supply leading to less opportunistic and other common ailments. These are the only postulates which in a general sense contribute to a good healthy body.
An important issue that requires evaluation is genetic differences in the Indian population vis-à-vis Caucasians, African and American population. We also recommend further studies on the subject to substantiate and bring out new determinants on this issue in the Indian setting.
In conclusion, a small subset of zidovudine treated patients may experience symptoms of fatigue, myalgia, reduced endurance and exercise intolerance, which may represent early signs of zidovudine-induced mitochondrial myopathy (due to an energy shortage within the muscle fibers even when muscle strength is still normal). This may be a cause of constraint for its use in western population. However, zidovudine is a well-tolerated drug in our experience in Indian subjects, as regards zidovudine-induced myopathy. Further studies in Indian population are recommended.
Source of Support: Nil,
Conflict of Interest: None declared.
References
- Mechanisms of zidovudine-induced mitochondrial toxicity and myopathy. Pharmacology. 2008;82:83-8.
- [Google Scholar]
- Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitor-induced mitochondrial toxicity. Clin Ther. 2000;22:685-708.
- [Google Scholar]
- Early features of zidovudine-associated myopathy: Histopathological findings and clinical correlations. Acta Neuropathol. 1995;90:1-6.
- [Google Scholar]
- Zidovudine-induced mitochondrial disorder with massive liver steatosis, myopathy, lactic acidosis and mitochondrial DNA depletion. J Hepatol. 1999;30:156-60.
- [Google Scholar]
- Clinical, histological and molecular reversibility of zidovudine myopathy. J Neurol Sci. 1998;159:226-8.
- [Google Scholar]
- Mitochondrial myopathy associated with chronic zidovudine therapy in AIDS. Q J Med. 1993;86:5-15.
- [Google Scholar]
- Myopathy associated with zidovudine use in HIV-1 infection. Int Conf AIDS. 1993;9:465.
- [Google Scholar]






